Neonatal Weight: No fire without smoke (particles)
Babies who are born with low birthweight (less than 2,500 g) or very low birthweight (less than 1,500g) are at risk of early postnatal complications, developmental problems during childhood, and additional diseases throughout their lifetime. Low birthweight is unfortunately very common, especially in low- and middle-income countries, where over 90% of low birth-weight babies are born (Blencowe et al., 2019). Because low birthweight is a global public health issue of major importance, the World Health Organization has made reducing its incidence one of its 2025 global targets. This is also a United Nations Sustainable Development Goal (World Health Organization, 2014).
While poverty – with associated malnutrition and infections – is likely the greatest cause of low birthweight in low- and middle-income countries, exposure to toxic agents in the environment can also contribute. Exposure to air pollution has previously been linked to both low birthweight and premature births (Li et al., 2017), especially air pollution due to particulate matter measuring less than 2.5 microns in diameter (PM2.5). By comparison, the diameter of a human hair is about 60 microns. PM2.5 particles are small enough to be inhaled deep into the lungs, where they can injure the delicate air sacs, and even cross into the bloodstream and cause issues in other organs. Most of the research on exposure to PM2.5 and low birthweight focuses on urban air pollution rather than on landscape fire smoke, which includes wildfires, deforestation fires, and burning of agricultural crop residues.
Climate change and deliberate deforestation have both contributed to a marked increase in large landscape fires in recent years (Hantson et al., 2017). The smoke from these fires contains many toxic agents, including PM2.5, and can lead to poor air quality over wide regions downwind from the fires. Now in eLife, Tao Xue from the Peking University Health Science Center and colleagues from the Peking Union Medical College, the University of Science and Technology Beijing, Tsinghua University and Zheijang University – with Jiajianghui Li and Tianjia Guan as joint first authors – report on how PM2.5 from landscape fires contributes to low birthweight in low and middle-income countries (Li et al., 2021).
Li et al., 2021 collected information on almost 228,000 babies born between 2000 and 2014 in 54 low- and middle-income countries. Birthweight data and individual characteristics came from demographic and health Surveys. Exposure to landscape fire PM2.5 during pregnancy was assessed through a sophisticated approach known as a chemical transport model (Xue et al., 2021), which was evaluated by comparing it to another well-known satellite-based approach (van Donkelaar et al., 2016). A chemical transport model uses information about pollutant emissions and atmospheric conditions to model the levels of different air pollutants, while the satellite approach uses satellite imagery to approximate the concentration of particles in the air. Li et al. then used a study design called a ‘sibling-matched case-control’, where babies are compared to their less exposed siblings, to study the association between landscape fire PM2.5 and birthweight, including the specific categories of low and very low birthweight. This design allows researchers to control for many of the complex factors that affect birthweight, including genetics, socioeconomic status, and quality of local health care, which can be difficult to measure.
Li et al. found that exposure to landscape PM2.5 during pregnancy meant babies had lower birthweights on average, and also that more babies met the criteria for low birthweight and very low birthweight (Figure 1). The association was stronger for female babies, first-born babies, and babies born to unemployed mothers. Moreover, babies born to families that had already had children with low average birthweights were most at risk. Li et al. controlled for multiple factors that might have led to bias in their results, including maternal age, child sex, multiple births, non-landscape fire PM2.5, birth order, temperature, humidity, month and year of birth, and country. Much of the effect of landscape fire PM2.5 was found in Sub-Saharan Africa, where approximately half of the babies were born, suggesting that other factors may have larger contributions to lower birthweights in other regions.
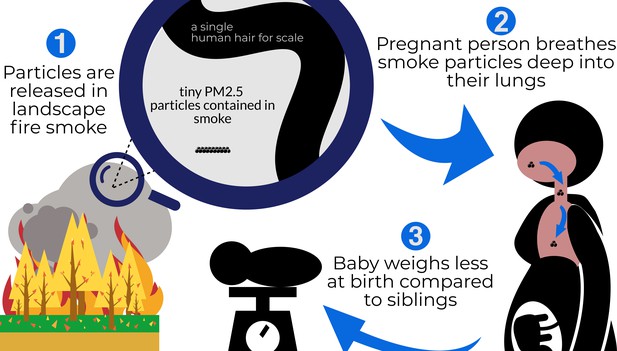
Schematic representation of Li et al.'s findings.
From the left, clockwise: (1) Landscape fires release particulate matter into the air, including particles measuring less than 2.5 microns in diameter (PM2.5). A magnifying glass shows how much smaller these particles are compared to the width of a strand of human hair (these particles are 2.5 microns or smaller; a hair is 60 microns) (2) Breathing in these tiny particles during pregnancy draws the particles deep into the lungs, where they lead to irritation and inflammation and enter the bloodstream affecting other organs, as well as the growing fetus. (3) When the baby is born, it weighs less than its siblings, who were exposed to lower levels of PM2.5 during pregnancy.
This study has many strengths, including a large study population, information on individual maternal characteristics, a sophisticated approach to assessing landscape fire PM2.5 exposure, and careful data analysis. The results are consistent with several smaller studies from high-income countries (Holstius et al., 2012; Cândido da Silva et al., 2014; Abdo et al., 2019). Perhaps the most impactful contribution of Li et al. is the focus on exposure to landscape fire PM2.5 in low and middle-income countries.
Despite its strengths, the study has several limitations. The survey data used did not have information on the date of conception, so a uniform nine month pregnancy period was used in the analysis. However, babies with low birthweights sometimes have shorter gestations, which would mean the exposure to landscape fire PM2.5 could have been underestimated for these babies. Another limitation is that the study relies on the mothers’ memory of the babies’ birthweights, rather than measurement. This could be an issue because recollection of birthweight can be influenced by how ill or healthy a child has been.
Exposure to landscape fire smoke frequently occurs in low-resource settings, which already have many risk factors for low birthweight. For this reason, it is important to implement policies to reduce the additional risk secondary to PM2.5 from landscape fires. Such policies include the promotion of alternative agricultural practices to reduce deforestation and burning of crop residue; better forest management to decrease fuel buildup; and climate change mitigation actions that incentivize clean transportation and renewable power generation. According to Li et al.’s results, these measures could help mitigate low birthweights due to PM2.5 in low to middle-income countries where these particles are mostly released by landscape fires, rather than other types of air pollution.
References
-
Impact of wildfire smoke on adverse pregnancy outcomes in Colorado, 2007-2015International Journal of Environmental Research and Public Health 16:E3720.https://doi.org/10.3390/ijerph16193720
-
Birth weight following pregnancy during the 2003 Southern California wildfiresEnvironmental Health Perspectives 120:1340–1345.https://doi.org/10.1289/ehp.1104515
-
Global estimates of fine particulate matter using a combined geophysical-statistical method with information from satellites, models, and monitorsEnvironmental Science & Technology 50:3762–3772.https://doi.org/10.1021/acs.est.5b05833
Article and author information
Author details
Publication history
Copyright
© 2021, Holm and Balmes
This article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use and redistribution provided that the original author and source are credited.
Metrics
-
- 332
- views
-
- 44
- downloads
-
- 0
- citations
Views, downloads and citations are aggregated across all versions of this paper published by eLife.
Download links
Downloads (link to download the article as PDF)
Open citations (links to open the citations from this article in various online reference manager services)
Cite this article (links to download the citations from this article in formats compatible with various reference manager tools)
Further reading
-
- Epidemiology and Global Health
- Genetics and Genomics
Burden of stroke differs by region, which could be attributed to differences in comorbid conditions and ethnicity. Genomewide variation acts as a proxy marker for ethnicity, and comorbid conditions. We present an integrated approach to understand this variation by considering prevalence and mortality rates of stroke and its comorbid risk for 204 countries from 2009 to 2019, and Genome-wide association studies (GWAS) risk variant for all these conditions. Global and regional trend analysis of rates using linear regression, correlation, and proportion analysis, signifies ethnogeographic differences. Interestingly, the comorbid conditions that act as risk drivers for stroke differed by regions, with more of metabolic risk in America and Europe, in contrast to high systolic blood pressure in Asian and African regions. GWAS risk loci of stroke and its comorbid conditions indicate distinct population stratification for each of these conditions, signifying for population-specific risk. Unique and shared genetic risk variants for stroke, and its comorbid and followed up with ethnic-specific variation can help in determining regional risk drivers for stroke. Unique ethnic-specific risk variants and their distinct patterns of linkage disequilibrium further uncover the drivers for phenotypic variation. Therefore, identifying population- and comorbidity-specific risk variants might help in defining the threshold for risk, and aid in developing population-specific prevention strategies for stroke.
-
- Epidemiology and Global Health
- Evolutionary Biology
Several coronaviruses infect humans, with three, including the SARS-CoV2, causing diseases. While coronaviruses are especially prone to induce pandemics, we know little about their evolutionary history, host-to-host transmissions, and biogeography. One of the difficulties lies in dating the origination of the family, a particularly challenging task for RNA viruses in general. Previous cophylogenetic tests of virus-host associations, including in the Coronaviridae family, have suggested a virus-host codiversification history stretching many millions of years. Here, we establish a framework for robustly testing scenarios of ancient origination and codiversification versus recent origination and diversification by host switches. Applied to coronaviruses and their mammalian hosts, our results support a scenario of recent origination of coronaviruses in bats and diversification by host switches, with preferential host switches within mammalian orders. Hotspots of coronavirus diversity, concentrated in East Asia and Europe, are consistent with this scenario of relatively recent origination and localized host switches. Spillovers from bats to other species are rare, but have the highest probability to be towards humans than to any other mammal species, implicating humans as the evolutionary intermediate host. The high host-switching rates within orders, as well as between humans, domesticated mammals, and non-flying wild mammals, indicates the potential for rapid additional spreading of coronaviruses across the world. Our results suggest that the evolutionary history of extant mammalian coronaviruses is recent, and that cases of long-term virus–host codiversification have been largely over-estimated.






