Genomic landscape of lymphatic malformations: a case series and response to the PI3Kα inhibitor alpelisib in an N-of-1 clinical trial
Abstract
Background:
Lymphatic malformations (LMs) often pose treatment challenges due to a large size or a critical location that could lead to disfigurement, and there are no standardized treatment approaches for either refractory or unresectable cases.
Methods:
We examined the genomic landscape of a patient cohort of LMs (n = 30 cases) that underwent comprehensive genomic profiling using a large-panel next-generation sequencing assay. Immunohistochemical analyses were completed in parallel.
Results:
These LMs had low mutational burden with hotspot PIK3CA mutations (n = 20) and NRAS (n = 5) mutations being most frequent, and mutually exclusive. All LM cases with Kaposi sarcoma-like (kaposiform) histology had NRAS mutations. One index patient presented with subacute abdominal pain and was diagnosed with a large retroperitoneal LM harboring a somatic PIK3CA gain-of-function mutation (H1047R). The patient achieved a rapid and durable radiologic complete response, as defined in RECIST1.1, to the PI3Kα inhibitor alpelisib within the context of a personalized N-of-1 clinical trial (NCT03941782). In translational correlative studies, canonical PI3Kα pathway activation was confirmed by immunohistochemistry and human LM-derived lymphatic endothelial cells carrying an allele with an activating mutation at the same locus were sensitive to alpelisib treatment in vitro, which was demonstrated by a concentration-dependent drop in measurable impedance, an assessment of cell status.
Conclusions:
Our findings establish that LM patients with conventional or kaposiform histology have distinct, yet targetable, driver mutations.
Funding:
R.P. and W.A. are supported by awards from the Levy-Longenbaugh Fund. S.G. is supported by awards from the Hugs for Brady Foundation. This work has been funded in part by the NCI Cancer Center Support Grants (CCSG; P30) to the University of Arizona Cancer Center (CA023074), the University of New Mexico Comprehensive Cancer Center (CA118100), and the Rutgers Cancer Institute of New Jersey (CA072720). B.K.M. was supported by National Science Foundation via Graduate Research Fellowship DGE-1143953.
Clinical trial number:
NCT03941782
Editor's evaluation
The study examines the genomic landscape of a patient cohort of lymphatic malformations (LMs) through next-generation sequencing and immunocytochemistry. The authors identified actionable driver mutations in the P13KCA and NRAS genes. The study enhances our understanding of the genetic architecture of the otherwise disfiguring LMs in people.
https://doi.org/10.7554/eLife.74510.sa0Introduction
Vascular anomalies, including lymphatic malformations (LMs), are usually diagnosed in children or young individuals and they can present as either isolated lesions or as part of somatic or congenital syndromes. Here, the term lymphatic malformation is used to include the clinicopathologic continuum of benign tumors of lymphatic origin (https://rarediseases.org/rare-diseases/lymphatic-malformations), including cystic lymphangioma, kaposiform lymphangiomatosis (KLM), and macro/microcystic LM. In general, LMs are managed by sclerotherapy, laser, or surgical interventions when there is an indication for therapy (Perkins et al., 2010). In certain cases, LMs can attain large sizes or involve critical locations, which poses treatment challenges such as the possibility of disfigurement. Genomic sequencing has demonstrated a somatic clonal origin for a number of nonmalignant growth conditions including LMs. Activating PIK3CA mutations have been reported in most pediatric patients with isolated or syndromic LMs (Luks et al., 2015). This finding has led to the use of mammalian target of rapamycin (mTOR) inhibitors for systemic therapy of unresectable LMs, given that mTOR is a molecule downstream of the PI3K pathway (Fruman et al., 2017). However, only a subset of patients responded, and the treatment can have substantial side effects. PI3K inhibitors have also been recently approved by the FDA for treatment of adults and children with severe manifestations PIK3CA-related Overgrowth Spectrum (termed PROS) who require systemic therapy, but the efficacy of alpelisib in isolated sporadic LMs is not at all clear. Activating NRAS mutations have been described in a subset of LM known as KLM (Barclay et al., 2019). KLM belong to a group of complex lymphatic anomalies that exhibit histologic and clinical features distinguishing them from classic LM. It is not as yet clear which oncogenic drivers, if any, are present in LMs with wild-type PIK3CA and NRAS alleles.
To define the spectrum of genomic alterations and lesions present in LMs, here we have analyzed a patient cohort of LMs (n = 30 cases) assayed by clinical-grade genomic sequencing. Pathogenic activating mutations in PIK3CA and NRAS were the most common genetic alterations found. Strikingly, the PIK3CA and NRAS mutations were mutually exclusive with NRAS mutations being greatly enriched in LMs with kaposiform morphology. We have also performed an N-of-1 trial of the PI3Kα inhibitor alpelisib in a young man with an activating PIK3CA point mutation, presenting with a giant (unresectable) retroperitoneal and pancreatic LM, who had a dramatic and prolonged response to the drug lasting years, and we present confirmatory translational correlates in vitro.
Materials and methods
Genomics and DNA sequencing
Request a detailed protocolHybrid-capture DNA sequencing targeting exons of at least 324 cancer genes and select introns of 36 genes were performed on the patient samples; a subset (n = 2) were also analyzed with plus RNA sequencing of 265 genes to improve rearrangement detection. A total of 30 patient samples were sequenced with either the DNA-only assay (n = 28; Foundation One CDx, Foundation Medicine; Cambridge, MA) or the DNA + RNA assay (n = 2; Foundation One Heme, Foundation Medicine; Cambridge, MA).
Immunohistochemistry
Request a detailed protocolImmunohistochemistry (IHC) was performed on formalin-fixed, deparaffinized, 5-µm-thick sections mounted on charged slides. Antibodies to P-AKT (Ser473) and P-6S (Ser240/Ser244) were obtained from Cell Signaling Technology, Danvers, MA. Diaminobenzidine was used as the chromogen and hematoxylin as the counterstain. All stages of staining were carried out on an automated system (Ventana Discovery Research Instrument; Ventana, Tucson, Arizona). Positive and negative controls were appropriately reactive. A surgical pathologist with subspecialty interest in musculoskeletal pathology (T.J.B.) interpreted the results.
Lymphatic malformation-lymphatic endothelial cell sensitivity to alpelisib in vitro
Request a detailed protocolLymphatic malformation-lymphatic endothelial cells (LM-LECs) were maintained as described (Boscolo et al., 2015) and negative for mycoplasma at the time of these studies. Mycoplasma test was performed using the MycoAlert Mycoplasma Detection Kit (Cat # LT07-218, Lonza) following the manufacturer’s instructions. Real-time analysis of cell viability was performed by using the xCELLigence system RTCA SP (ACEA Biosciences). Briefly, 5 × 103 LM-LECs per well were seeded in an E-Plate 96 (ACEA Biosciences) and cell proliferation was recorded hourly. When the cells reached the exponential growth phase, new media containing alpelisib at 1, 3, 10, 30, or 100 nM was added and alpelisib cytotoxic effect was recorded hourly. IC50 was calculated by using the dose–response curve function available in the xCELLigence software Version 2.0. Cell index (%) reflects cell viability.
Clonogenic survival assays
Request a detailed protocolFor the clonogenic survival assay, the LM-LECs were trypsinized, counted, and plated in complete growth media on 6-well plates (Falcon) (400 cells/well). Seven days later, alpelisib (at the empirically determined IC50 from a standard calibration curve) was added in duplicate wells. After 24 or 48 hr of incubation, cells were fixed and stained in 50% methanol in water containing 0.3% crystal violet to facilitate counting of colonies (≥50cells).
Statistics
All values are expressed as mean with error bars expressed as standard deviation. For comparison between untreated (negative), dimethyl sulfoxide control, and alpelisib-treated LM-LEC cells, the ordinary one-way analysis of variance and Tukey’s multiple comparisons test with a single pooled variance were used. Statistical analysis was performed using the GraphPad Prism 7.0d software (GraphPad Software Inc, San Diego, CA). Fisher’s exact test was used for categorical data, owing to the sizes of the cohorts. A two-tailed p value of <0.05 was considered to be statistically significant.
Study approval
Request a detailed protocolApproval for this study, including a waiver of informed consent and Health Insurance Portability and Accountability Act waiver of authorization, was obtained from the Western Institutional Review Board (IRB; protocol #20152817). A single-institution personalized clinical protocol to treat the patient with the experimental PI3Kα inhibitor alpelisib was scientifically reviewed by the Protocol Review and Monitoring Committee (PRMC) and approved by the local Institutional Review Board (IRB) of the University of New Mexico Comprehensive Cancer Center. The study (NCT03941782) was conducted in accordance with the protocol, Good Clinical Practice guidelines, and the provisions of the Declaration of Helsinki. CARE reporting guidelines were also used for this patient (Gagnier et al., 2013). The index patient signed an informed written consent form.
Results
Mutational landscape and histopathology of LMs
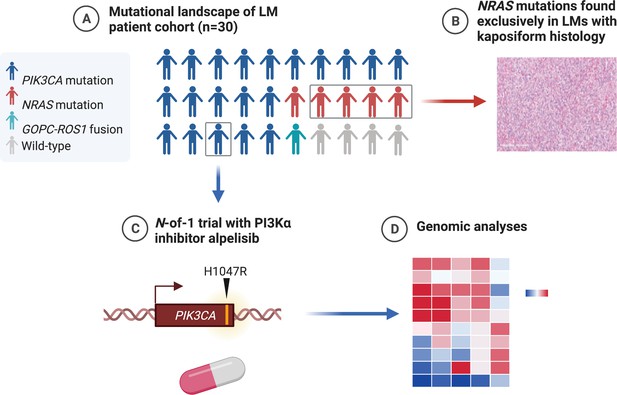
A set of 30 cases of LMs (from 30 individual patients) were assayed with genomic profiling at Foundation Medicine, Inc (Cambridge, MA). Twenty-eight cases were sequenced using hybrid-capture next-generation sequencing (NGS) targeting exons of 300 + cancer genes and select introns of 36 genes. Two other cases were sequenced using hybrid-capture based DNA sequencing targeting exons of 406 + cancer genes and select introns of 36 genes, plus RNA sequencing of 265 genes for rearrangement calling. The patients were predominantly pediatric age (median 9-year-old; range, 1- to 45-year-old), with a slight female predominance (17 females, 57%–13 males, 43%). Seven patients had a documented history of prior treatment with an mTOR inhibitor, such as sirolimus. Seven patients (23%) had documentation of clinical diagnoses of overgrowth syndromes including Congenital Lipomatous Overgrowth with Vascular, Epidermal, and Skeletal anomalies (termed CLOVES), Klippel–Trenaunay syndrome, and phosphatase and tensin homolog (PTEN)-like hamartoma syndrome at the time of testing. Twelve patients (40%) had multifocal disease and eight patients had involvement of bone and visceral sites (Table 1). Expert histopathological review showed that only four (13%) had kaposiform morphology, while 26 (87%) had conventional histology. The estimated histopathologic purity ranged from 10% to 70% (median 20%).
Clinical and histological features of lymphatic malformation cohort.
| Patient | Age(years) | Sex | Submitted clinical syndrome | Localized vs. multifocal | Location of LM(s) | Specimen type | LM histology | PIK3CA or NRAS alteration | % VAF |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 9 | M | CLOVES | Multifocal | Superficial soft tissues | Excision | Conventional | PIK3CA E542K | 14 |
| 2 | 4 | F | Localized | Superficial soft tissues | Excision | Conventional | PIK3CA E542K | 7 | |
| 3 | 1 | F | Localized | Superficial soft tissues | Excision | Conventional | PIK3CA H1047R | 11 | |
| 4 | 17 | M | Localized | Superficial soft tissues | Excision | Conventional | PIK3CA H1047R | 4 | |
| 5 | 18 | M | Localized | Superficial soft tissues | Excision | Conventional | PIK3CA H1047L | 4 | |
| 6 | 8 | F | Klippel–Trenaunay | Localized | Superficial soft tissues | Excision | Conventional | PIK3CA H1047R | 9 |
| 7 | 9 | M | Localized | Visceral | Core biopsy | Conventional | PIK3CA E545K | 7 | |
| 8 | 3 | F | Localized | Superficial soft tissues | Excision | Conventional | PIK3CA C420R | 5 | |
| 9 | 23 | M | Localized | Visceral | Incisional biopsy | Conventional | PIK3CA H1047R | 4 | |
| 10 | 16 | F | PTEN-like hamartoma | Localized | Superficial soft tissues | Excision | Conventional | PIK3CA H1047R | 3 |
| 11 | 3 | F | CLOVES | Multifocal | Superficial soft tissues | Excision | Conventional | PIK3CA E545K | 12 |
| 12 | 1 | M | Multifocal | Superficial soft tissues | Excision | Conventional | PIK3CA H1047R | 2 | |
| 13 | 4 | F | Localized | Superficial soft tissues | Excision | Conventional | PIK3CA E542K | 6 | |
| 14 | 5 | M | Localized | Superficial soft tissues | Excision | Conventional | PIK3CA H1047R | 5 | |
| 15 | 1 | F | Localized | Superficial soft tissues | Excision | Conventional | PIK3CA E545K | 1 | |
| 16 | 14 | F | Multifocal | Visceral | Excision | Conventional | PIK3CA C420R | 14 | |
| 17 | 2 | F | CLOVES | Multifocal | Superficial soft tissues | Excision | Conventional | PIK3CA C420R | 38 |
| 18 | 16 | F | CLOVES | Localized | Superficial soft tissues | Excision | Conventional | PIK3CA E453K | 32 |
| 19 | 10 | F | CLOVES | Multifocal | Superficial soft tissues | Excision | Conventional | PIK3CA H1047L | 15 |
| 20 | 9 | M | Localized | Superficial soft tissues | Excision | Conventional | PIK3CA H1047R | 5 | |
| 21 | 9 | F | Multifocal | Visceral | Excision | Kaposiform | NRAS Q61R | 5 | |
| 22 | 8 | M | Multifocal | Superficial soft tissues | Excision | Kaposiform | NRAS Q61R | 5 | |
| 23 | 9 | F | Multifocal | Visceral | Excision | Kaposiform | NRAS Q61R | 1 | |
| 24 | 45 | M | Multifocal | Visceral | Core biopsy | Conventional | NRAS Q61R | 6 | |
| 25 | 10 | F | Localized | Superficial soft tissues | Core biopsy | Kaposiform | NRAS Q61R | 14 | |
| 26 | 17 | M | Multifocal | Superficial soft tissues | Excision | Conventional | WT | NA | |
| 27 | 24 | M | Localized | Bone | Core biopsy | Conventional | WT | NA | |
| 28 | 3 | M | Multifocal | Superficial soft tissues | Excision | Conventional | WT | NA | |
| 29 | 11 | F | Localized | Superficial soft tissues | Excision | Conventional | WT | NA | |
| 30 | 9 | F | Localized | Superficial soft tissues, bone | Biopsy | Conventional | WT | NA |
-
CLOVES – congenital lipomatous overgrowth, vascular anomalies, epidermal nevi, and skeletal anomalies; NA – not applicable; VAF – variant allele frequency of PIK3CA or NRAS.
Mutational profiling showed that these LMs had uniformly low tumor mutational burden (median, zero mutations/Mb; range, 0–2.6 mutations/Mb), and none had evidence of microsatellite instability. The most common mutations were activating mutations in PIK3CA, seen in 20 (67%), and activating NRAS mutations, seen in 5 (17%) (Figure 1A, B). The PIK3CA mutations included hotspot mutations in both the helical domain and the kinase domain (Samuels et al., 2004). The NRAS mutations all altered the known hotspot at residue glutamine 61 (Q61) in the phosphorylation binding loop. Of the five patients (17%) with no alterations in PIK3CA and NRAS, one case (Patient #29; Table 1) had an activating GOPC–ROS1 fusion (Figure 1C) with a ROS1 missense point mutation. Similar GOPC–ROS1 fusions have been reported in pediatric gliomas in the setting of microdeletion of chromosome 6q228, and have also been found in adult lung cancer (Drilon et al., 2021).
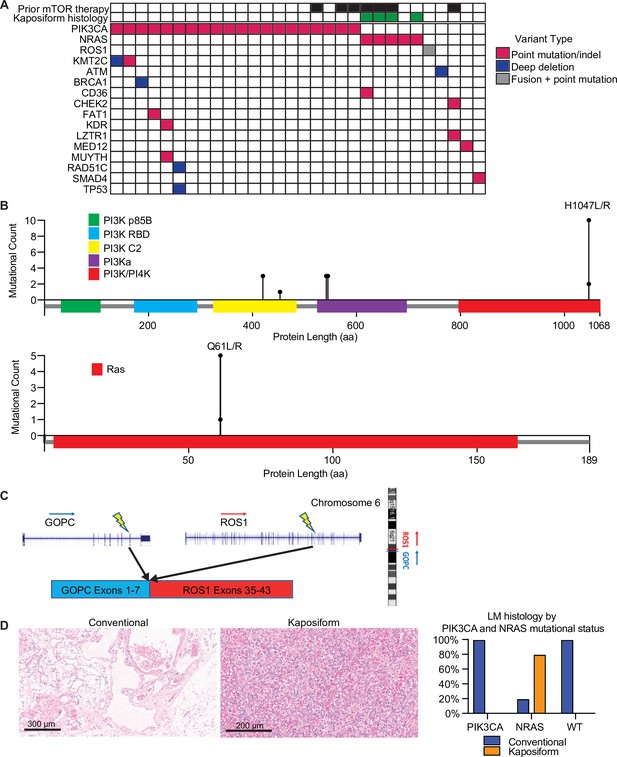
Mutational landscape and histopathology of lymphatic malformations (LMs).
(A) Oncoprint showing mutational landscape of 30 LM samples sequenced. (B) Lollipop plot showing spectrum of PIK3CA and NRAS mutations in this cohort. (C) Schema showing details of GOPC–ROS1 fusion identified in an NRAS and PIK3CA wild-type LM. (D) Representative histologic images for LMs with conventional and kaposiform histology. The relative frequencies of PIK3CA and NRAS mutations in the two histologic variants are plotted.
The variant allele frequencies (VAFs) of the PIK3CA and NRAS mutations were relatively low (median, 6%; range, 1–38%), compatible with relatively low histopathologic estimated percentage of tumor nuclei (%TN) to overall cellular nuclei (median, 20%; range, 10–70%). These results suggest that the PIK3CA and NRAS mutations were likely clonal, but in the setting of relatively low tumor purity in the specimens.
Enrichment of NRAS mutations in LMs with kaposiform features
Histopathological analysis of the lesions by an board-certified dermatopathologist (J.Y.T.) identified that four (13%) of the analyzed specimens had kaposiform histopathological features with highly cellular, clustered, or sheet-like, proliferation of spindled lymphatic cells admixed with dilated thin-walled lymphatic vessels (Figure 1D). The remaining 26 lesions (87%) had conventional histopathological features of classic LM, with proliferation of dilated, thin-walled lymphatic vessels with or without luminal proteinaceous material. Lymphatic phenotype of the cells was confirmed by immunopositivity for PROX1 or D2-40, by report. Of the conventional histology LM cases (n = 26), 20 (77%) had a PIK3CA mutation, while 1 (4%) had a NRAS mutation, and 5 (19%) were wild-type for both genes, including a single case with a GOPC–ROS1 genetic fusion. Notably, all four cases of LM with kaposiform features had an activating NRAS mutation, consistent with enrichment of NRAS mutation (p = 0.00018) and lack of PIK3CA mutation in this histology (p = 0.0046). The lone NRAS-mutant LM with conventional histology was a small core needle-biopsy specimen of a large visceral tumor, raising the possibility that the histopathologic features of the sampled tissue may not have been representative of the entire lesion due to the histologic spatial heterogeneity often seen in LMs with kaposiform histology. Additional histopathologic features were assessed, including altered adipose tissue, muscularized blood vessels, vascular endothelial cell atypia, and inflammation; no statistical significance was identified between the four NRAS-mutant LM cases and the remainder of the patient cohort.
Case report and N-of-1 clinical trial results
One of the conventional histology LMs was a 23-year-old male with no significant medical or family history who presented with subacute abdominal pain (Patient #9, Table 1). He was hospitalized and his exam revealed a distended abdomen that was tender to palpation. A computed tomography exam revealed a large solid mass based on the retroperitoneal area and the pancreas (Figure 2A), and a neoplastic process was suspected. A core needle biopsy was attempted but yielded no definitive tissue diagnosis. An open laparoscopic surgical biopsy was performed and revealed a vascular tumor with features of a giant retroperitoneal and pancreatic LM (Figure 2D, E). After discussing a surgical approach, the patient and the surgical team decided not to proceed due to the complexity of surgical resection and associated risks. The tissue was submitted for NGS to identify potential biomarkers for targeted therapy.
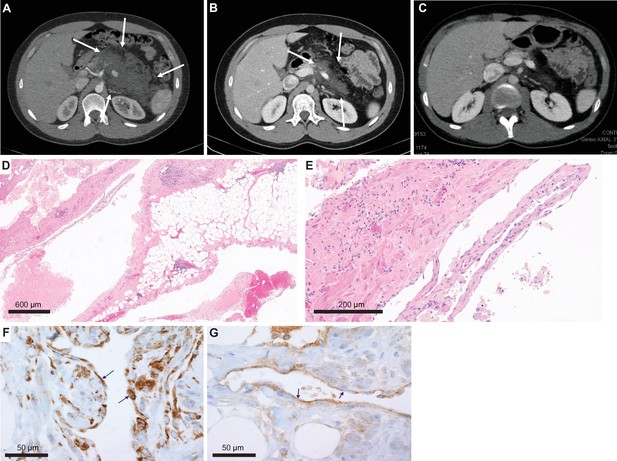
Imaging and histological analysis of lymphatic malformation (LM) patient.
(A) Baseline CT abdomen scan at the time of presentation demonstrating a large retroperitoneal/pancreatic LM. (B) CT abdomen scan 6 weeks after the initiation of alpelisib. (C) CT abdomen scan 1 year into the trial. (D, E) Hematoxylin and eosin (H&E)-stained photomicrographs of the LM showing dilated lymphatic channels percolating through visceral fat and associated patchy lymphocytic inflammation (×4 and ×20, respectively). (F) Immunohistochemistry utilizing an anti-P-6S antibody demonstrates PI3Ka pathway activation within the channels’ lining cells. (G) Anti-P-AKT positivity in the lining endothelium of lymphatic channels as well.
Clinical-grade sequencing of the biopsy sample from Patient #9 uncovered a single activating point mutation in PIK3CA (H1047R). All other genes in the panel were wild-type except for another unit of the PI3K complex (PIK3C2B) that showed a variant (R458Q) of unknown significance (VUS). To confirm activation of the PI3Kα pathway, we performed IHC staining of the downstream targets (P-AKT and P-6S), and, as predicted, these phosphorylation events were detected in the lining cells of the abnormal lymphatic channels (Figure 2F, G).
Based on the genomic profile, we designed and offered this young man a single-patient (N-of-1) personalized clinical trial of the PI3Kα inhibitor alpelisib (NCT03941782), which at the time was still investigational (non-FDA approved). Screening procedures included an echocardiogram that revealed an ejection fraction (EF) of 47%. A cardiac MRI confirmed a low EF with no infiltrative process or other abnormalities. Paradoxically, the patient was completely asymptomatic from a cardiac standpoint and he was able to run two miles on a daily basis. We hypothesized that the decreased EF, in the absence of accompanying clinical signs or symptoms of heart failure, was likely artefactual due to hemodynamic changes related to the very large circulatory volume sequestration in his abdomen.
The patient was started on alpelisib daily dose of 350 mg orally (Juric et al., 2018) and he reported regression of his abdominal bulge within a few days. He reported no adverse events and was closely monitored for hyperglycemia. Repeated echocardiogram 2 months later showed normalization of the EF. A CT scan of the abdomen done 6 weeks into the trial revealed remarkable shrinkage of the LM (Figure 2B). Follow-up CT scans showed progressive reduction until complete response at 1 year of trial initiation (Figure 2C). The patient continued to do well on maintenance alpelisib for 2 years with no evidence of progression. After 2 years, alpelisib was discontinued due to theoretical concerns about long-term adverse impact on vascular homeostasis. Unfortunately, the mass recurred after a few weeks so the patient was resumed on alpelisib with a second deep partial response, which is still ongoing for over 3 years.
Alpelisib inhibits primary PI3Kα-mutant LM-derived endothelial cells
We have also investigated the concentration-dependent effects of alpelisib on LM-LECs isolated from a surgically resected specimen (Boscolo et al., 2015). Targeted sequencing of DNA from LM-LECs identified a somatic missense mutation in PIK3CA (H1047L), the same locus altered in our alpelisib-treated patient and the site of half of the PIK3CA alterations in the LM cohort studied (Table 1). In addition, a nonsense mutation of the regulatory PI3K unit PIK3R3 (R309*) was also detected in the CD31-positive LM-LECs and CD31-negative nonendothelial cells isolated from the same LM, indicating its germline origin (Boscolo et al., 2015). We investigated the effect of alpelisib on the growth of LM-LECs and a concentration-dependent response curve was observed (Figure 3). The IC50 of alpelisib against LM-LECs was empirically determined in vitro to be 4.72 × 10−9 M at 24 hr. This in vitro translational model confirms the sensitivity of LM-derived human cells containing a target H1047R/L mutation to alpelisib.
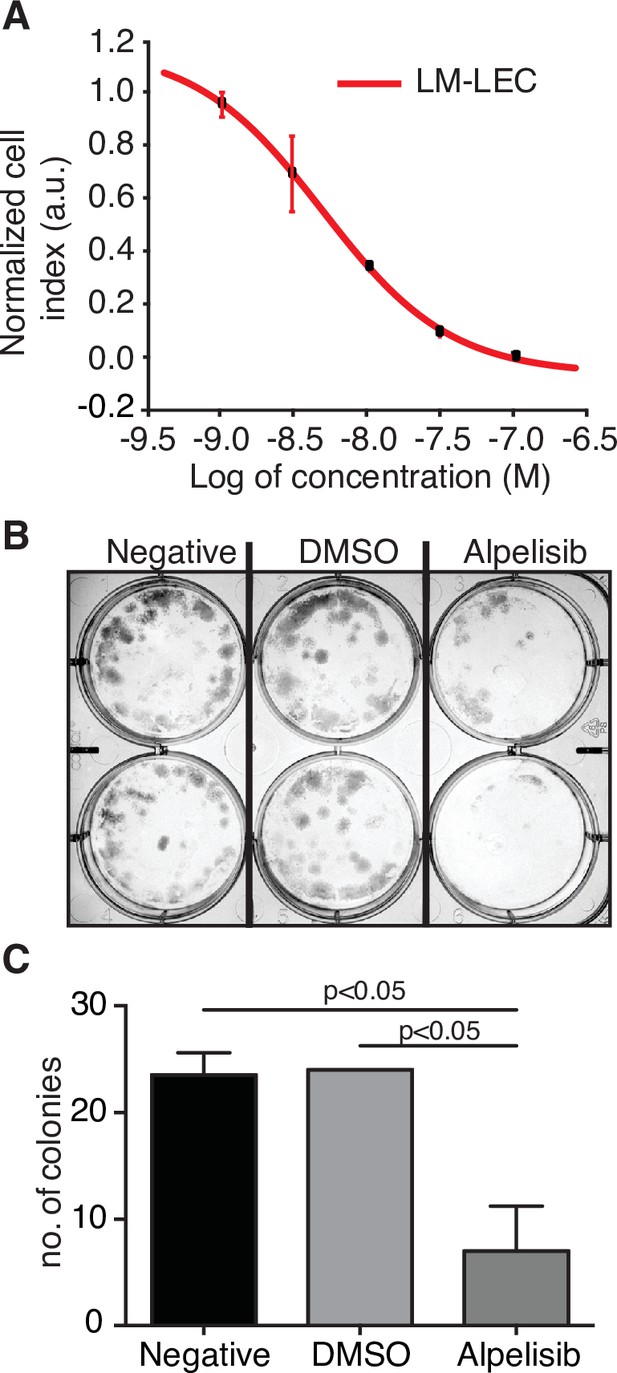
Alpelisib reduces lymphatic malformation-lymphatic endothelial cell (LM-LEC) viability.
(A) Logarithmic dose–response curve of alpelisib was performed using the xCELLigence RTCA system. 1, 3, 10, 30, and 100 nM (n = 5 replicates) of alpelisib were used to determine the concentration–response curve. The alpelisib half maximal inhibitory concentration (IC50) was calculated for LM-LEC at 24 hr after treatment as 4.72 × 10−9 M. Error bars are shown as mean +/- standard deviation (SD), which was automatically calculated for each data point by the xCELLigence RTCA system software (Version 2.0) based on five replicates per drug concentration. (B) Illustrative picture of LM-LEC clonogenic plaques at 24 hr after alpelisib treatment (4.72 × 10−9 M). Negative, no treatment; dimethyl sulfoxide (DMSO), vehicle control. Experiments were performed two times with similar results. LM-LEC colonies were stained with crystal violet (0.3%). (C) Colony count 24 hr after alpelisib treatment (4.72 × 10−9 M; n = 2 wells/condition). Error bars are shown as mean +/- SD calculated by GraphPad Prism by determining the square root of variance for each data point deviation relative to the mean.
Refined genomic and sequencing analyses
We performed whole-genome sequencing (WGS) on paired LM/germline DNA from our index patient to explore the mutational profile beyond the genes that were probed in the Clinical Laboratory Improvement Amendments (CLIA)-approved clinical sequencing assay. The PIK3CA H1047R mutation was identified with a VAF of 11%. This finding is consistent with the ≤10% rate of mutant cells, and low tumor cellularity of LMs with PIK3CA mutation (Luks et al., 2015). Few other somatic coding mutations were identified in the LM tissue (Supplementary file 1).
To gain further molecular mechanistic insight, we have also performed RNA-seq studies to identify gene expression patterns within the LM sample from our index patient compared to normal tissue (Figure 4). RNA-seq data of biopsy samples from Patient #9 (n = 2 samples; Figure 4A, Group A) were compared to several normal human control tissue samples from bladder, colon, kidney, and salivary gland (n = 4, one sample per each tissue; Figure 4A, Group B). There is little difference between the two LM samples, but, by using an arbitrary cutoff of at least twofold up or down with adjusted p values of 0.05 or less, we identified 668 upregulated and 850 downregulated genes. The heatmap summarizes the results of the differential gene expression analysis; 125 genes are shown. The volcano plot summarizes the distribution of genes that were differentially expressed (Figure 4B). Here, the vertical axis shows the p value and the horizontal axis shows the fold-change. The genes that were more than twofold changed and had an adjusted p value less than 0.05 are shaded red. Similar numbers of genes were up- and downregulated. Several of the most highly induced genes, CHI3L1, GPX1, PLIN1, PLIN4, and JAK3, have been linked to enhanced growth or cell survival in other tumor types (Cheng et al., 2019; Qiu et al., 2018; Sirois et al., 2019; Vadivel et al., 2021; Zhang et al., 2020). Finally, a preliminary Gene Ontogeny (GO) analysis (Subramanian et al., 2007) of Patient #9 LM revealed enrichment of mRNA of genes involved in vascular development, cell motility, inflammatory response, positive regulation of response to stimuli, blood vessel morphogenesis, among others; notably, the kinase JAK3 gene was one of the highest expression mRNAs in the LMs compared to normal tissue controls.
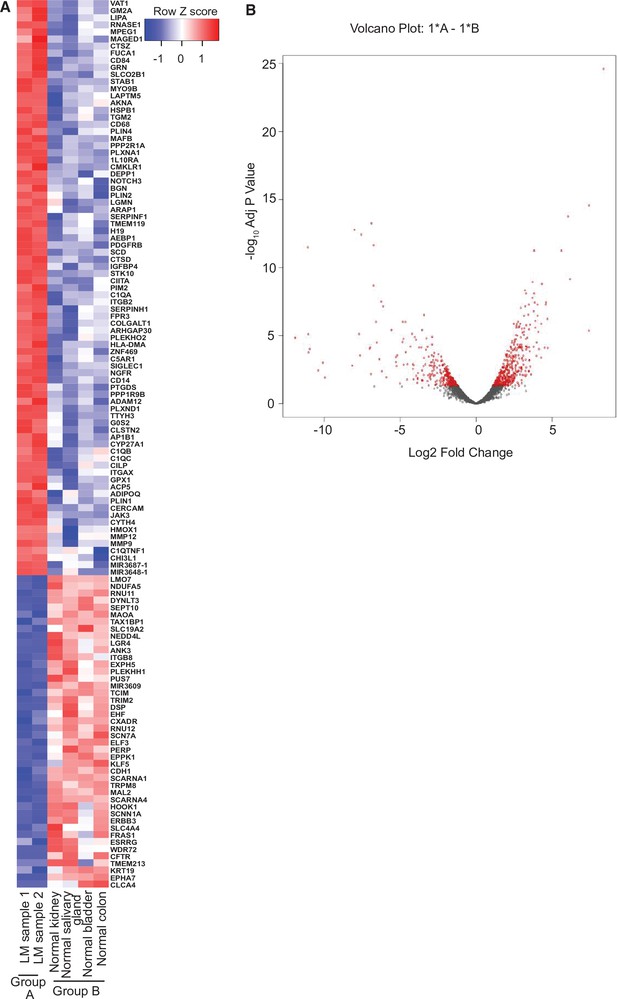
RNA-seq analysis of lymphatic malformation (LM) samples from index patient (#9).
(A) The heatmap summarizes the results of the differential gene expression analysis. Up- and downregulated genes are shaded red and blue, respectively. (B) The volcano plot summarizes the distribution of genes that were differentially expressed. The vertical axis shows the p value and the horizontal shows the fold-change. The genes that were more than twofold changed and had an adjusted p value less than 0.05 are shaded red. Similar numbers of genes were up- or downregulated.
Discussion
Here, we report the mutational landscape of a patient cohort of LMs (n = 30 cases) which underwent comprehensive genomic profiling. We have confirmed prior reports that hotspot activating mutations in PIK3CA are common driver events in these lesions, seen in 20 (67%) of these cases. Interestingly, NRAS mutations were seen in an additional five (17%) cases and were particularly enriched in LMs with a kaposiform histopathology. This finding supports previous studies that have shown that LMs with kaposiform features likely represent a distinct entity (KLA) (Barclay et al., 2019; Croteau et al., 2014). KLM have distinct clinical, histologic, and genomic features. Clinically, they are more likely to occur in young patients and commonly present as generalized processes with involvement of the mediastinum, pleura, and pericardium. Histologically, they are composed of highly cellular sheet-like and nodular proliferations of spindle cells, reminiscent of Kaposi sarcoma (Croteau et al., 2014). Unlike Kaposi sarcoma, the tumor cells lack immunopositivity for human herpesvirus-8 (HHV-8) latency-associated nuclear antigen (LANA). Genomically, recent studies have shown they tend to harbor somatic activating alterations in NRAS (Barclay et al., 2019Barclay et al., 2019). As a caveat, for the one NRAS-mutant LM with classic histology, the histologic classification was based on a small biopsy, and it is certainly possible that kaposiform histology was present in the large visceral LM but not captured by the limited sampling by core needle biopsy. Importantly, three of the five patients (60%) with NRAS-mutant LMs had failed treatment with sirolimus prior to NGS. There are reports that some NRAS-mutant LMs may respond to treatment with MEK inhibitors (Dummer et al., 2017), suggesting this may be an option for LMs with kaposiform features.
Of the five cases without either PIK3CA or NRAS mutations, all of classic histology, a single case had a known pathogenic in-frame GOPC–ROS1 genetic fusion predicted to have an intact ROS1 kinase domain and thus potentially function as the driver. Similar GOPC–ROS1 fusions have been seen in pediatric gliomas and adult lung cancers and may be sensitive to ROS1 inhibitors (Davare et al., 2018; Drilon et al., 2021). These data suggest that most LMs may have a potentially actionable driver mutation, with PIK3CA mutations dominating LMs with conventional histology and NRAS mutations predominantly or exclusively seen in the minor subset of LMs with kaposiform features. It is possible that the other NRAS and KRAS wild-type LMs may also have oncogenic alterations in other members of the PIK3CA or MAPK signaling pathway members that were not profiled by targeted sequencing strategies. We appreciate that one limitation of our study is that the cohort presented in Table 1 was established from clinical information provided by the ordering physicians early in the course of diagnostic investigation. Therefore, we cannot rule out that the working clinical diagnosis and/or pathologic diagnoses were refined after genomic analyses without transmission of these data to the reference laboratory that supplied the cohort data. Comprehensive NGS analysis of LMs with PIK3CA and NRAS wild-type may be required to identify any potential actionable driver mutations. In patients without solid LM tissue available for NGS, liquid biopsy—or NGS performed on circulating tumor DNA (ctDNA) in peripheral blood—may be a possible solution for LMs, which are innately associated with the vascular system and thus potentially ‘shedding’ ctDNA into the peripheral blood.
To illustrate the potential for therapeutic intervention of the target mutations identified, we performed an N-of-1 trial of alpelisib in one young adult index patient with a giant retroperitoneal and pancreatic LM with conventional histological features and a gain-of-function H1047R somatic PIK3CA mutation. Our index patient experienced a rapid, complete, and durable clinical response with this small molecule PI3Kα inhibitor. Given the high frequency of PIK3CA mutations in pediatric LMS (Luks et al., 2015), this finding suggests that alpelisib may be highly effective for systemic, nonsurgical treatment approach to this class of disorders. Furthermore, the lack of toxicity to alpelisib in our case is promising in terms of a potential future treatment of young patients with LMs. Our patient did not experience increases in glucose levels, consistent with reported lack of alpelisib-induced hyperglycemia in most pediatric patients with PROS (Venot et al., 2018; Mayer et al., 2017). In this prior series, only one patient developed new-onset hyperglycemia and this was controlled by dietary modification (Venot et al., 2018). These findings suggest that the effect of alpelisib on inducing hyperglycemia might perhaps be less of a concern in younger patients, who may have more robust glucose homeostasis, compared with older patients who may already have subclinical insulin resistance.
Ultimately, we decided to hold alpelisib after 2 years of complete radiological response, and unfortunately the LM relapsed but the patient still achieved a major partial response on the second challenge with alpelisib. This result suggests that PI3Kα inhibitors do not completely eradicate all LM-initiating cells, and they may need to be given long term (in our young index patient case, perhaps over decades) in PIK3CA-mutant LMs for sustained control. This class of drugs can also be envisioned to be utilized in a neoaduvant approach to render large cases resectable. Our patient declined surgery after initial response and he continues on alpelisib for several years. Acquired resistance mechanisms to PI3Kα inhibitors have been reported, due to other associated compensatory or bypassing mutations such as ones involving RAS oncogene (Janku et al., 2014) or PTEN tumor suppressor gene (Juric et al., 2017), and these may conceivably arise in these patients with longer follow-up over time. Deftly balancing the potential benefits of continuing treatment with the potential for drug resistance mechanisms will require monitoring for both actionable known and novel mutations through NGS of LM tissue samples or liquid biopsy.
In a series of pediatric patients with LMs, Luks et al. identified PIK3CA gene mutations in patients with sporadic LMs in 16 out of 17 patients (94%) or syndromic LMs such as the Klippel–Trenaunay syndrome in 19 out of 21 patients (90%), fibro-adipose vascular anomaly in 5 out of 8 patients (63%), along with the CLOVES syndrome in 31 out of 33 patients (94%) (Luks et al., 2015). H1047R was one of the top 2 most frequently encountered hotspot mutations in this series. Venot et al. reported a single arm clinical trial of alpelisib in 19 patients with pediatric PROS including CLOVES (Venot et al., 2018). Alpelisib treatment-induced clinical responses in all patients, including improvement of cardiac EF as seen in our index patient. Of note, alpelisib-induced responses in patients who did not respond to prior treatment with mTOR inhibitors, such as rapamycin, similar to observations in KRAS-mutant oncology patients (Di Nicolantonio et al., 2010), similar to the recent findings of Delestre et al. (Delestre et al., 2021). Small clinical series have shown that mTOR inhibition can induce responses in a subset of unselected advanced LMs, with observed response rates of ~50–60% (Freixo et al., 2020). The on driver-oncoprotein activity, higher response rates, and tolerability suggest alpelisib may be more effective than mTOR inhibitors in this setting. It is tempting to speculate that a wide variety of PIK3CA-mutant somatic overgrowth conditions (Hucthagowder et al., 2017) may be amenable to medical treatment with FDA-approved PI3Kα inhibitors, either as neoadjuvant treatment in potentially resectable cases, or as primary treatment in unresectable cases (Juric et al., 2018; Juric et al., 2017; Bendell et al., 2012; Hong et al., 2012; Juric et al., 2015). Since our submission, the FDA-approved alpelisib for pediatric and adult patients with PROS, and several clinical trials are underway to assess safety, efficacy, and quality-of-life with alpelisib in patients with a PROS diagnosis (e.g., NCT04589650, NCT04085653, NCT04980833, and NCT05294289), demonstrating the swiftness of efforts to address this clinical need.
Furthermore, in our WGS analysis, we identified only a few somatic variants within protein-coding genetic sequences (Supplementary file 1) beyond what was reported in the cancer gene panel (Table 1). The low frequency of somatic mutations is consistent with findings in other low-grade pediatric tumors (Akhavanfard et al., 2020). In addition to detecting the PIK3CA H1047R mutation, this WGS confirmed the variant detected by the cancer gene panel in the PIK3C2B gene and demonstrated that it was germline. Although this in PIK3C2B variant has not been characterized and may be a benign polymorphism, this finding raises the issue of whether other alterations in the pathway may cooperate with activating mutations of PIK3CA to induce cell proliferation. The low VAF driver mutations in tissue derived from LMs is likely due to the fact that most pathological tissue is composed of reactive stromal elements while the clonal cells represent a relatively small portion (presumably the lymphatic channel-lining endothelial cells). Consistent with this observation, in the alpelisib-treated index patient, we observed most intense activation of the PI3Kα pathway in these lymphatic channel-lining endothelial cells (Figure 2F, G). The high representations of pathways associated with vascular development, cell motility, inflammatory response, positive regulation of response to stimuli, blood vessel morphogenesis in our gene expression analysis are consistent with a mechanistic hypothesis that most of the lesion represents an intense reactive response to the (presumably) clonal LM-LECs, although the appropriate comparator control tissues for these lesions is not clear.
Evidence is accumulating that a variety of ‘nonmalignant’ syndromes associated with abnormal tissue growth may be driven by underlying alterations in classic oncogenes (Mustjoki and Young, 2021). PIK3CA mutations are seen not only in LMs but other vascular anomalies, highlighting the role of PIK3CA activation in angiogenesis, lymphangiogenesis, and vascular neoplasms (Castel et al., 2016; Castillo et al., 2016; Limaye et al., 2015; Ren et al., 2021). Endometriosis, uterine fibroids, and seborrheic keratoses all have been found to harbor mutations in cancer-related genes (Rafnar et al., 2018; Gallagher et al., 2019; Fritsche et al., 2018; Sanders et al., 2018; Anglesio et al., 2017). These findings suggest that targeted therapies being developed for invasive cancers may also be active in proliferative lesions that are not classified as invasive cancers that harbor the targeted alteration.
In summary (Figure 5), we find that the majority of LMs have driver mutations that are potentially targetable. LMs with classic histology mostly have PIK3CA mutations that may respond to alpelisib. LMs with kaposiform histopathology are enriched in NRAS mutations, and studies are required to determine if these may respond to clinically available MEK inhibitors. LMs that are wild-type for PIK3CA and NRAS may have other actionable alterations, such as the GOPC–ROS1 fusion seen in our series and may require more comprehensive genomic analyses to identify them. Systemic treatment with targeted therapy aimed at the driver mutation in LMs may be an option for some patients who are not controlled by surgery and other conventional treatments.
Graphical summary of the mutations found in genomic analysis of lymphatic malformation (LM) patient cohort (created with BioRender.com).
(A) The majority of LMs have driver mutations that are potentially targetable. (B) LMs with NRAS mutations had kaposiform histopathology. (C) An N-of-1 clinical trial is reported in a patient with a targetable PIK3CA mutation. (D) Comprehensive genomic analyses may reveal further actionable molecular insights.
Data availability
NGS data for this study were generated at Foundation Medicine, Inc on U.S. patients profiled during routine clinical care. Approval for this study, including a waiver of informed consent and Health Insurance Portability and Accountability Act waiver of authorization, was obtained from the Western Institutional Review Board (protocol #20152817). Due to potential for identifiability, patient-level alteration data are not available. However, extensive data supporting the findings of this study are available in Table 1 and Figure 1A.
References
-
Cancer-Associated Mutations in Endometriosis without CancerThe New England Journal of Medicine 376:1835–1848.https://doi.org/10.1056/NEJMoa1614814
-
A somatic activating NRAS variant associated with kaposiform lymphangiomatosisGenetics in Medicine 21:1517–1524.https://doi.org/10.1038/s41436-018-0390-0
-
Phase I, dose-escalation study of BKM120, an oral pan-Class I PI3K inhibitor, in patients with advanced solid tumorsJournal of Clinical Oncology 30:282–290.https://doi.org/10.1200/JCO.2011.36.1360
-
Somatic PIK3CA mutations as a driver of sporadic venous malformationsScience Translational Medicine 8:332–342.https://doi.org/10.1126/scitranslmed.aaf1164
-
Somatic activating mutations in Pik3ca cause sporadic venous malformations in mice and humansScience Translational Medicine 8:332–343.https://doi.org/10.1126/scitranslmed.aad9982
-
Kaposiform lymphangiomatosis: a distinct aggressive lymphatic anomalyThe Journal of Pediatrics 164:383–388.https://doi.org/10.1016/j.jpeds.2013.10.013
-
Alpelisib administration reduced lymphatic malformations in a mouse model and in patientsScience Translational Medicine 13:614.https://doi.org/10.1126/scitranslmed.abg0809
-
Deregulation of the PI3K and KRAS signaling pathways in human cancer cells determines their response to everolimusThe Journal of Clinical Investigation 120:2858–2866.https://doi.org/10.1172/JCI37539
-
ROS1-dependent cancers - biology, diagnostics and therapeuticsNature Reviews. Clinical Oncology 18:35–55.https://doi.org/10.1038/s41571-020-0408-9
-
Efficacy and safety of sirolimus in the treatment of vascular anomalies: A systematic reviewJournal of Vascular Surgery 71:318–327.https://doi.org/10.1016/j.jvs.2019.06.217
-
Association of Polygenic Risk Scores for Multiple Cancers in a Phenome-wide Study: Results from The Michigan Genomics InitiativeAmerican Journal of Human Genetics 102:1048–1061.https://doi.org/10.1016/j.ajhg.2018.04.001
-
The CARE guidelines: consensus-based clinical case reporting guideline developmentBMJ Case Reports 2013:bcr2013201554.https://doi.org/10.1136/bcr-2013-201554
-
Somatic Activating PIK3CA Mutations Cause Venous MalformationAmerican Journal of Human Genetics 97:914–921.https://doi.org/10.1016/j.ajhg.2015.11.011
-
Lymphatic and other vascular malformative/overgrowth disorders are caused by somatic mutations in PIK3CAThe Journal of Pediatrics 166:1048–1054.https://doi.org/10.1016/j.jpeds.2014.12.069
-
Somatic Mutations in “Benign” DiseaseThe New England Journal of Medicine 384:2039–2052.https://doi.org/10.1056/NEJMra2101920
-
Lymphatic malformations: review of current treatmentOtolaryngology--Head and Neck Surgery 142:795–803.https://doi.org/10.1016/j.otohns.2010.02.026
-
High frequency of mutations of the PIK3CA gene in human cancersScience (New York, N.Y.) 304:554.https://doi.org/10.1126/science.1096502
-
The Genetics of Seborrheic Dermatitis: A Candidate Gene Approach and Pilot Genome-Wide Association StudyThe Journal of Investigative Dermatology 138:991–993.https://doi.org/10.1016/j.jid.2017.11.020
-
GSEA-P: a desktop application for Gene Set Enrichment AnalysisBioinformatics (Oxford, England) 23:3251–3253.https://doi.org/10.1093/bioinformatics/btm369
Article and author information
Author details
Funding
Levy-Longenbaugh Fund
- Renata Pasqualini
- Wadih Arap
Hugs for Brady Foundation
- Shridar Ganesan
National Cancer Institute (P30CA072720)
- Renata Pasqualini
- Shridar Ganesan
- Wadih Arap
National Science Foundation (DGE-1143953)
- Brian K Mannakee
National Cancer Institute (P30CA023074)
- Montaser F Shaheen
National Cancer Institute (P30CA118100)
- Scott A Ness
The funders had no role in study design, data collection, and interpretation, or the decision to submit the work for publication.
Acknowledgements
We thank Dr. Kathryn J Brayer for technical assistance and Dr. Helen Pickersgill (Life Science Editors) for professional manuscript editing services.
Ethics
Clinical trial registration NCT03941782.
Approval for this study, including a waiver of informed consent and Health Insurance Portability and Accountability Act waiver of authorization, was obtained from the Western Institutional Review Board (IRB; protocol #20152817). A single-institution personalized clinical protocol to treat the patient with the experimental PI3Ka inhibitor alpelisib was scientifically reviewed by the Protocol Review and Monitoring Committee (PRMC) and approved by the local Institutional Review Board (IRB) of the University of New Mexico Comprehensive Cancer Center. The study (NCT03941782) was conducted in accordance with the protocol, Good Clinical Practice guidelines, and the provisions of the Declaration of Helsinki. The index patient signed an informed written consent form.
Copyright
© 2022, Shaheen, Tse et al.
This article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use and redistribution provided that the original author and source are credited.
Metrics
-
- 1,132
- views
-
- 222
- downloads
-
- 13
- citations
Views, downloads and citations are aggregated across all versions of this paper published by eLife.
Citations by DOI
-
- 13
- citations for umbrella DOI https://doi.org/10.7554/eLife.74510






