Contextual control of conditioned pain tolerance and endogenous analgesic systems
Figures
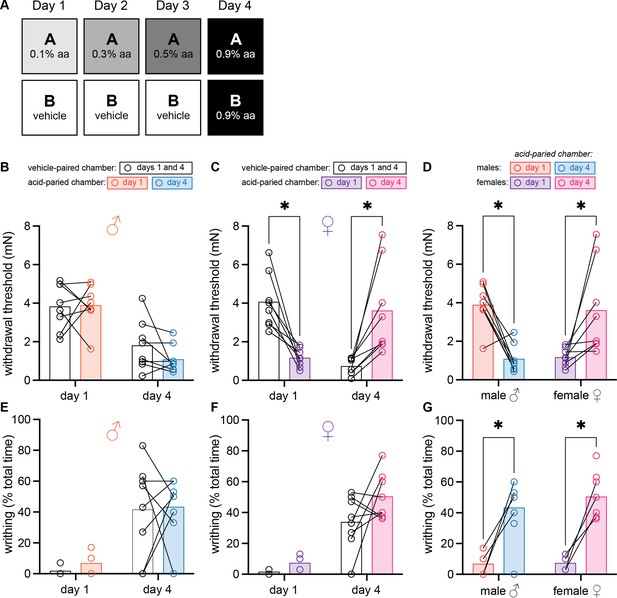
Female mice develop context-dependent analgesia after training with ascending doses of acetic acid.
(A) Experimental design depicting the within-subject procedure. In daily sessions, mice were given ascending doses of acetic acid in one physical chamber, Context A, and vehicle injections in a separate physical chamber, Context B. On the final day, mice received an injection of 0.9% acetic acid solution in each context. Writhing behaviors were assessed on days 1 and 4 for the first 30 min following injection. Hindpaw sensitivity was measured 45 min following injection on days 1 and 4. (B) von Frey withdrawal thresholds of male mice (n = 8) on days 1 and 4 of the paradigm. Acetic acid injection on day 1 (0.1%) had no effect on hindpaw mechanical sensitivity. Hindpaw mechanical sensitivity was similar in both contexts following 0.9% acetic acid injection on day 4. (C) von Frey withdrawal thresholds of female mice (n = 8) on days 1 and 4 of the paradigm. Acetic acid injection on day 1 (0.1%) induced hindpaw mechanical hypersensitivity (‘hyperalgesic descending control of nociception [DCN]’). Hindpaw mechanical sensitivity differed between the contexts following 0.9% acetic acid injection on day 4, however; females exhibited contextually mediated analgesia in the acid-paired chamber relative to the vehicle-paired chamber on day 4. (D) von Frey withdrawal thresholds of male and female mice replotted to highlight results in the acetic acid paired chamber. Male mice exhibit hyperalgesic DCN on day 4 relative to day 1. Female mice exhibit less hindpaw sensitivity following 0.9% acetic acid injection on day 4 than they did following 0.1% acetic acid injection on day 1 suggesting the development of conditioned analgesia. (E) Writhing behavior of male mice on days 1 and 4 of the paradigm. Writhing frequency was similar in both contexts on days 1 and 4. (F) Writhing behavior of female mice on days 1 and 4 of the paradigm. Writhing frequency was similar in both contexts on days 1 and 4. (G) Writhing behavior of male and female mice replotted to highlight results in acetic acid paired chamber. Both sexes exhibited more writhing behaviors following a 0.9% acid injection on day 4 than following a 0.1% acid injection on day 1.
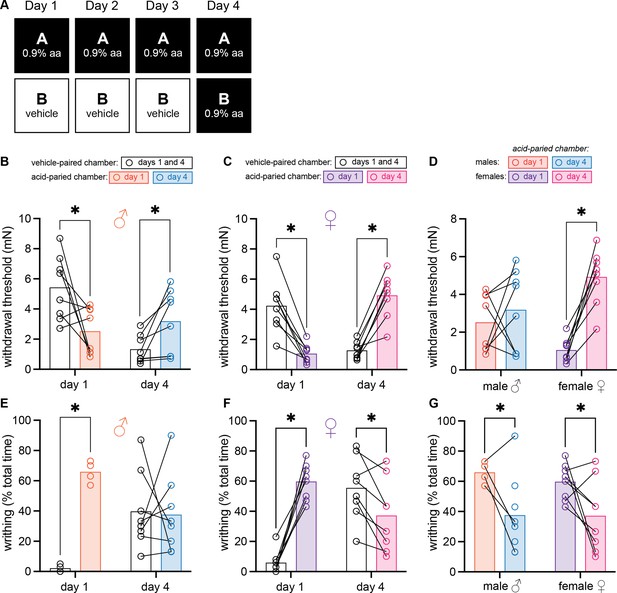
Female and male mice develop context-dependent analgesia after training with high doses of acetic acid.
(A) Experimental design depicting the within-subject procedure. In daily sessions, mice were given a 0.9% dose of acetic acid in one physical chamber, Context A, and vehicle injections in a separate physical chamber, Context B. On the final day, mice received an injection of 0.9% acetic acid solution in each context. Writhing behaviors were assessed on days 1 and 4 for the first 30 min following injection. Hindpaw sensitivity was measured 45 min following injection. (B) von Frey withdrawal thresholds of male mice (n = 8) on days 1 and 4 of the paradigm. Acetic acid injection on day 1 (0.9%) induced hindpaw mechanical hypersensitivity (‘hyperalgesic descending control of nociception [DCN]’). Hindpaw mechanical sensitivity differed between the contexts following 0.9% acetic acid injection on day 4, however; males exhibited contextually mediated analgesia in the acid-paired chamber relative to the vehicle-paired chamber on day 4. (C) von Frey withdrawal thresholds of female mice (n = 8) on days 1 and 4 of the paradigm. Acetic acid injection on day 1 (0.9%) induced hindpaw mechanical hypersensitivity (‘hyperalgesic DCN’). Hindpaw mechanical sensitivity differed between the contexts following 0.9% acetic acid injection on day 4, however; females exhibited contextually mediated analgesia in the acid-paired chamber relative to the vehicle-paired chamber on day 4. (D) von Frey withdrawal thresholds of male and female mice replotted to highlight results in the acetic acid paired chamber. Male mice exhibited similar levels of hindpaw mechanical sensitivity in the acid-paired chamber on days 1 and 4. Female mice exhibited less hindpaw sensitivity following 0.9% acetic acid injection on day 4 than they did following 0.9% acetic acid injection on day 1 suggesting the development of conditioned analgesia. (E) Writhing behavior of male mice on days 1 and 4 of the paradigm. On day 1, injection of 0.9% acid induced more writhing than injection of vehicle. Writhing frequency of male mice was similar in both contexts on day 4. (F) Writhing behavior of female mice on days 1 and 4 of the paradigm. On day 1, injection of 0.9% acid induced more writhing than injection of vehicle. Writhing frequency of female mice was lower in the acid-paired context on day 4 than in the vehicle-paired context. (G) Writhing behavior of male and female mice replotted to highlight results in acetic acid paired chamber. Both sexes exhibited less writhing on day 4 relative to day 1.
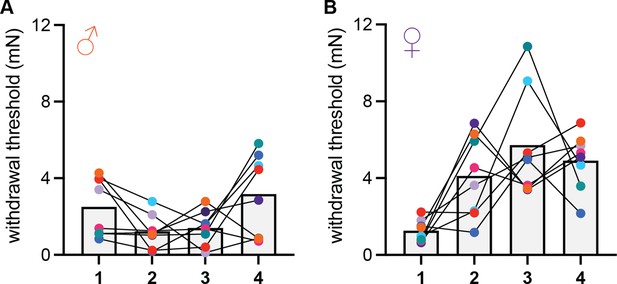
Time course of conditioned analgesia development in male and female mice.
(A) Hindpaw withdrawal thresholds of male mice in the acid-paired chamber on days 1–4. (B) Hindpaw withdrawal thresholds of female mice in the acid-paired chamber on days 1–4. Data points for individual subjects are color-coded and connected across days. All animals received an injection of 0.9% acetic acid 45 min prior to being tested each day.
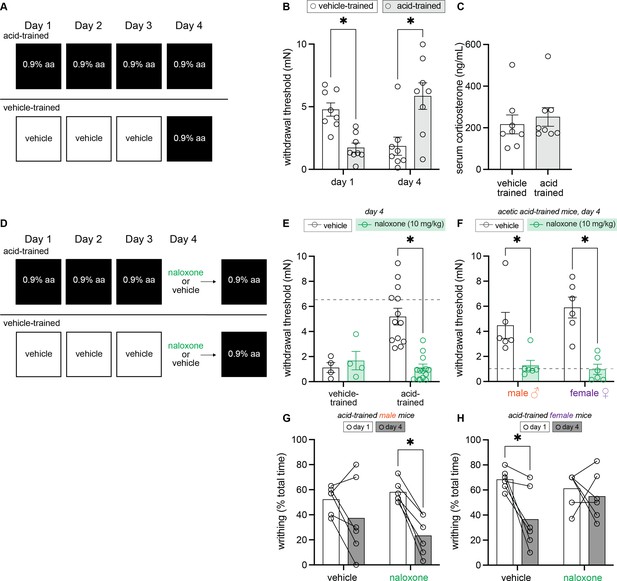
Endogenous opioid signaling, and not circulating corticosterone, is associated with contextually mediated conditioned analgesia.
(A) Experimental design depicting the between-subject design. Animals received an intraperitoneal (IP) injection of either acetic acid or vehicle for 3 days. On the fourth day, all animals were given an IP injection of 0.9% acetic acid. (B) von Frey withdrawal thresholds of male and female mice on days 1 and 4 of the paradigm. Animals in the vehicle-trained group (n = 8; 4 males, 4 females) have higher withdrawal thresholds than those injected with acid on day 1. On day 4, vehicle-trained mice (n = 8; 4 males, 4 females) exhibit ‘hyperalgesic descending control of nociception [DCN]’ and have lower withdrawal thresholds than acid-trained mice. Acid-trained mice exhibit ‘hyperalgesic DCN’ on day 1 and conditioned analgesia on day 4. (C) Results from ELISA corticosterone assay. Groups (n = 8; same mice from panel B) did not differ in their circulating cortisol levels 60 min following acid injection. (D) Experimental design depicting the between-subjects design. Animals received an IP injection of either acetic acid or vehicle for 3 days. On the fourth and final day, all animals were given an IP injection of acetic acid. However, half of the animals in each condition were pre-treated with naloxone, an opioid receptor antagonist, while the other half received vehicle. (E) von Frey withdrawal thresholds of male and female mice on day 4. Animals in the vehicle-trained groups (n = 4) showed mechanical hypersensitivity irrespective of naloxone pre-treatment, and animals in the acid-trained group (n = 12) exhibited conditioned analgesia that was blocked by naloxone. The dashed gray line represents the average withdrawal threshold of vehicle-trained mice on day 1. (F) von Frey withdrawal thresholds of acid-trained male (n = 6 per treatment group) and female (n = 6 per treatment group) mice replotted to examine sex differences on day 4. Both males and females showed increased mechanical hypersensitivity when pre-treated with naloxone, suggesting that contextually mediated analgesia is mediated by the endogenous opioid system. The dashed gray line indicates the average withdrawal threshold of vehicle-trained mice on day 4. (G) Writhing frequency of acid-trained male mice on days 1 and 4 of the paradigm. On day 4, naloxone pre-treated mice exhibited a decrease in writhing as compared to day 1. (H) Writhing frequency of acid-trained female mice on days 1 and 4 of the paradigm. On day 4, vehicle pre-treated mice exhibited a reduction in writhing frequency as compared to day 1. Naloxone pre-treatment prevented this decrease in writhing on day 4, suggesting the reduction observed in vehicle-treated animals is a form of conditioned analgesia.
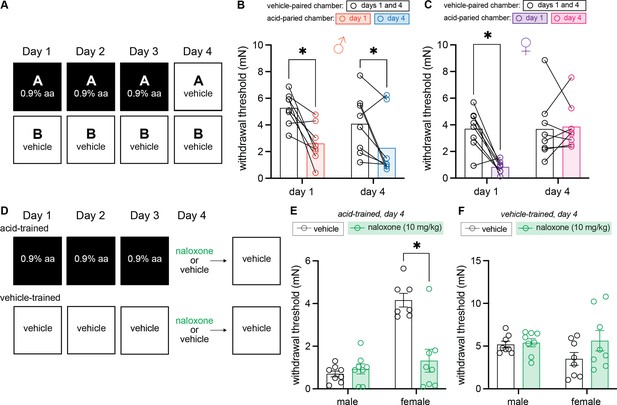
Males exhibit conditioned hypersensitivity and females exhibit conditioned analgesia in the absence of unconditioned stimulus (UCS) administration.
(A) Experimental design depicting the within-subject procedure. In daily sessions, mice were given a 0.9% dose of acetic acid in one physical chamber, Context A, and vehicle injections in a separate physical chamber, Context B. On the final day, mice received an injection of vehicle in each context. Hindpaw sensitivity was measured 45 min following injection on days 1 and 4. (B) von Frey withdrawal thresholds of male mice (n = 8) on days 1 and 4 of the paradigm. Acetic acid injection on day 1 (0.9%) induced hindpaw mechanical hypersensitivity (‘hyperalgesic descending control of nociception [DCN]’). Similar hindpaw mechanical hypersensitivity was observed in the acid-paired chamber on day 4, despite the fact that animals received a vehicle injection prior to being placed in this chamber. (C) von Frey withdrawal thresholds of female mice (n = 8) on days 1 and 4 of the paradigm. Acetic acid injection on day 1 (0.9%) induced hindpaw mechanical hypersensitivity (‘hyperalgesic DCN’). Hindpaw mechanical sensitivity did not differ between the contexts following vehicle injection on day 4. (D) Experimental design depicting the between-subject procedure. In daily sessions, mice were given a 0.9% dose of acetic acid or vehicle injection prior to being placed into one physical chamber. On the final day, mice received an injection of naloxone or vehicle prior to being placed into the same training context; no acid injection was performed on day 4. (E) von Frey withdrawal thresholds of acid-trained male (n = 8) and female (n = 7–8) mice on day 4 of the paradigm. Naloxone pre-treatment did not affect the hindpaw withdrawal thresholds of acid-trained male mice. Conversely, naloxone treatment decreased the hindpaw withdrawal thresholds of acid-trained female mice, suggesting conditioned recruitment of endogenous opioid systems in the training context. (F) Withdrawal thresholds of vehicle-trained male (n = 8) and female (n = 8) mice on day 4 of the paradigm. Naloxone pre-treatment had no effect on hindpaw sensitivity of male or female mice after 3 days of context training.
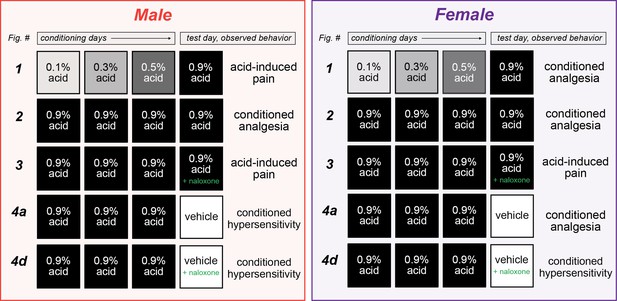
Summary of sexually divergent conditioned pain behaviors.
Graphical depiction of conditioning trials, test day manipulations, and subsequent behavior results. After training with high doses of acetic acid, male mice exhibited opioid-dependent conditioned analgesia when presented with the same noxious unconditioned stimulus (UCS) (row 2), and conditioned hypersensitivity when placed into the context where conditioning occurred (row 4a). Alternatively, female mice developed opioid-dependent conditioned analgesia after training with low (row 1) or high (row 2) doses of acetic acid. This conditioned analgesia was also observed when animals were placed into the context where conditioning occurred, even in the absence of the UCS (row 4a).





