Endurance exercise ameliorates phenotypes in Drosophila models of spinocerebellar ataxias
Figures
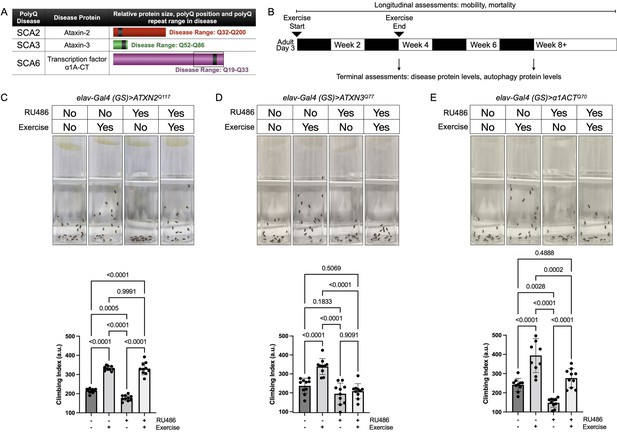
Endurance exercise differentially affects mobility in Drosophila models of spinocerebellar ataxia.
(A) Spinocerebellar ataxia (SCA) models used in this study. Box in SCA6 highlights the transcription factor, β1A-CT that is encoded through an internal ribosomal entry site of the β1A transcript. It is this transcript that we utilized for SCA6 model flies. (B) Timeline of endurance exercise program and assessment of physiology and disease protein levels. (C–E) Representative climbing speed images (upper panels) in Drosophila models of (C) SCA2, (D) SCA3 and (E) SCA6. Photos taken 2 s after inducing negative geotaxis response in 4-week-old flies, following endurance exercise completion. Bottom panels: quantification by ANOVA with Tukey’s post-hoc for significantly different groups; means ± SD. n = 10. Each individual datum depicts average week four climbing speed in arbitrary units (a.u.) for five vials of 20 flies.
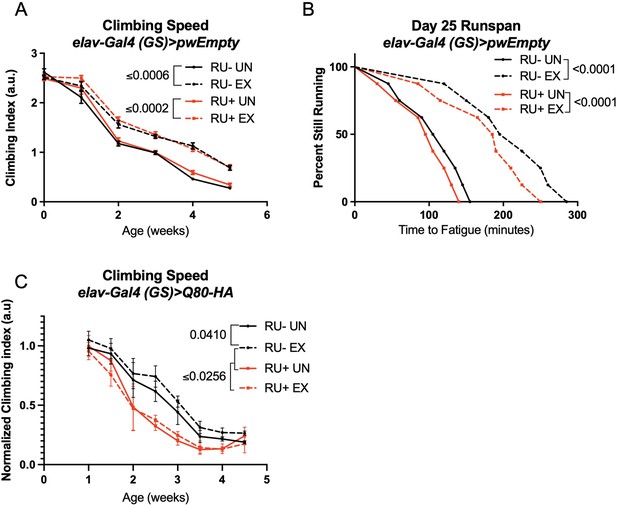
Endurance exercise improves climbing speed in uninduced background control flies.
(A, B) elav-Gal4 (GS)> pwEmpty vector flies improve (A) climbing speed and (B) endurance independent of RU486-feeding. (C) Flies expressing an isolated polyQ-80 repeat in adult neurons have lower climbing speed than age-matched, uninduced control flies and do not increase speed with exercise. Mobility and endurance experiments performed in triplicate. Each individual datum depicts average climbing speed for ≥5 vials of 20 flies, analyzed by 2-way ANOVA (climbing speed), or time for 80% of flies in an individual vial (n ≥ 8 vials of 20 flies) to reach exhaustion, analyzed by log rank (endurance). Error bars indicate ± SD. pwEmpty: pwalium10.moe host vector inserted into the chromosomal site attP2 of the fly and in the same genetic background as the SCA lines used in this study.
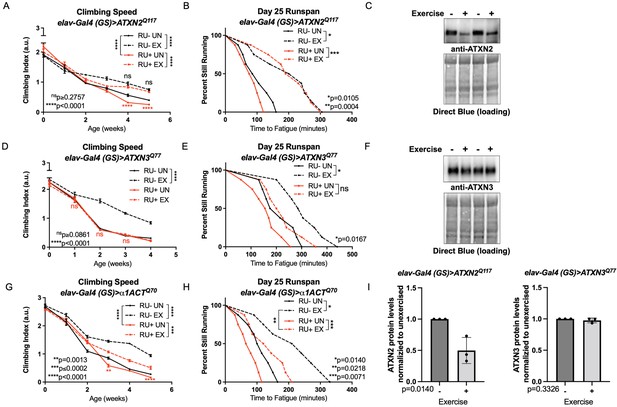
Endurance exercise differentially affects mobility and disease protein levels in Drosophila models of SCA.
(A) Flies ectopically expressing polyQ-expanded ATXN2 in adult neurons (RU+ UN) have lower climbing speed than unexercised, uninduced control flies (RU- UN) by adult week 4. Exercise fully rescues climbing speed to the level of exercised, uninduced control flies (compare RU- EX to RU+ EX). (B) Flies expressing polyQ-expanded ATXN2 in adult neurons (RU+) have similar endurance to uninduced control flies (RU-) whether exercised or not. (C) Exercise reduces ATXN2 protein levels in flies ectopically expressing polyQ-expanded ATXN2 in adult neurons, quantified in (I). (D, E) Flies ectopically expressing polyQ-expanded ATXN3 in adult neurons (RU+) have similar (D) climbing speed and endurance to uninduced, unexercised control flies and fail to improve either (D) climbing speed (E) endurance with exercise. (F) Exercise does not affect ATXN3 protein levels in flies expressing CAG-expanded ATXN3 in adult neurons, quantified in (I). (G) Flies ectopically expressing CAG-expanded β ACT in adult neurons (RU+) have lower climbing speed than unexercised, uninduced control flies (RU- UN) by adult week 3, and exercise partially rescues climbing speed, although not to the level of exercised, uninduced control flies. (H) Exercise improves endurance in flies expressing CAG-expandedα1ACT in adult neurons (RU+ EX), but not to the level of exercised, uninduced control siblings (compare RU- EX to RU+ EX). Mobility and endurance data are presented as representative experiments from triplicate biological replicates. Each individual datum depicts average climbing speed for ≥5 vials of 20 flies, analyzed by 2-way ANOVA (climbing speed), or time for 80% of flies in an individual vial (n ≥ 8 vials of 20 flies) to reach exhaustion, analyzed by log rank (endurance). Representative Western blots (five flies/lysate) from three biological repetitions, analyzed by ANOVA with Tukey’s post-hoc for significantly different groups. Error bars indicate ± SD.
-
Figure 2—source data 1
Uncropped, unedited blots from Figure 2C.
- https://cdn.elifesciences.org/articles/75389/elife-75389-fig2-data1-v3.zip
-
Figure 2—source data 2
Uncropped, unedited blots from Figure 2F.
- https://cdn.elifesciences.org/articles/75389/elife-75389-fig2-data2-v3.zip
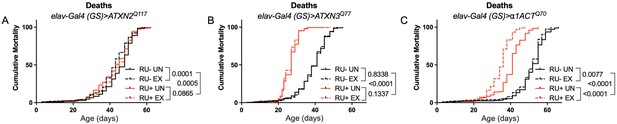
Expression of ATXN2 in adult neurons does not negatively impact lifespan.
Cumulative mortality in (A) elav-Gal4 (GS)> ATXN2Q117 flies is similar whether exercised or not (compare RU- UN to RU+ UN, and RU- EX to RU+ EX). (B) elav-Gal4 (GS)> ATXN3Q77 flies have reduced lifespan compared to uninduced controls, and exercise does not negatively affect either group. (C) elav-Gal4 (GS)>α1ACTQ70 flies have lower lifespan than uninduced controls, and exercise reduces lifespan further in this cohort. n ≥ 231, analyzed by log-rank tests.
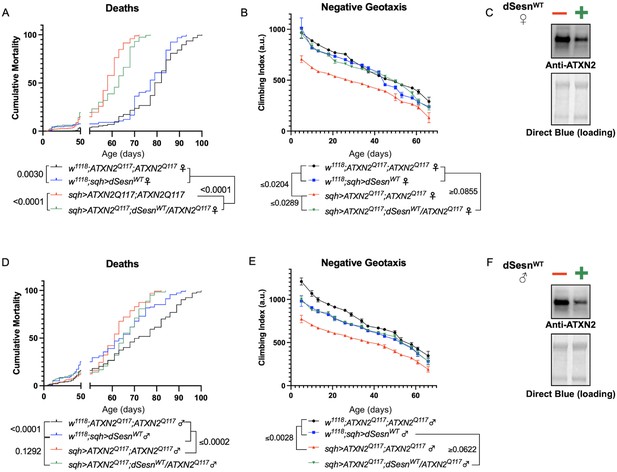
dSesn expression improves early death and low mobility in SCA2 flies, concurrent with reduction in disease protein.
Female (A, B) and male (D, E) flies ubiquitously expressing two copies of CAG-expanded ATXN2 (red lines) have early death (A, D) and lower climbing speed (B, E) than age-matched background control flies (blue and black lines). dSesn expression in flies also expressing polyQ-expanded ATXN2 (green lines) partially rescues early death (A, D) and fully rescues decreased mobility (B, E). ATXN2 protein levels are lower in both female (C) and male (F) flies ubiquitously expressing both dSesn and polyQ-expanded ATXN2. Survival and mobility experiments performed in triplicate. Individual survival experiments comprise ≥200 flies/genotype, scored every second day for death and analyzed by log-rank tests. Each individual climbing datum depicts average climbing speed for ≥5 vials of 20 flies, analyzed by 2-way ANOVA. Error bars indicate ± SD. Representative Western blots from five biological replicates (five flies per lysate), quantified in Figure 7.
-
Figure 4—source data 1
Uncropped, unedited blots from Figure 4C.
- https://cdn.elifesciences.org/articles/75389/elife-75389-fig4-data1-v3.zip
-
Figure 4—source data 2
Uncropped, unedited blots from Figure 4F.
- https://cdn.elifesciences.org/articles/75389/elife-75389-fig4-data2-v3.zip
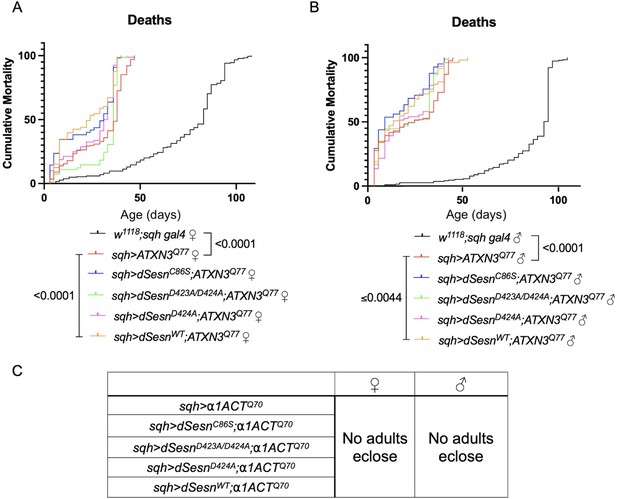
Ubiquitous dSesn expression fails to increase survival in flies expressing either polyQ-expanded ATXN3 or polyQ-expanded α1ACT.
Survival curves in female (A) and male (B) background control flies (black) or flies ubiquitously expressing polyQ-expanded ATXN3 and either wild-type dSesn (orange), dSesnC86S (blue), dSesnD424A (pink), or dSesnD423A/D424A (green). n ≥ 73, analyzed by log-rank tests. (C) No adults eclose in flies ubiquitously expressing polyQ-expanded α1ACT regardless of dSesn expression. Sufficient parental crosses set up for >400 flies per genotype.
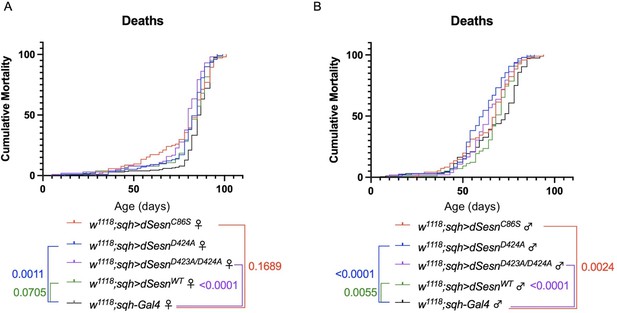
Ubiquitous dSesn expression in wild-type flies does not account for survival differences observed in flies expressing polyQ-expanded ATXN2.
Survival curves in female (A) and male (B) flies expressing either wild-type dSesn (green), dSesnC86S (red), dSesnD424A (blue), dSesnD423A/D424A (purple) or background control (black). Flies ubiquitously expressing dSesn mutations have similar or lower survival than flies ubiquitously expressing wild type dSesn. n ≥ 97, analyzed by log-rank tests.
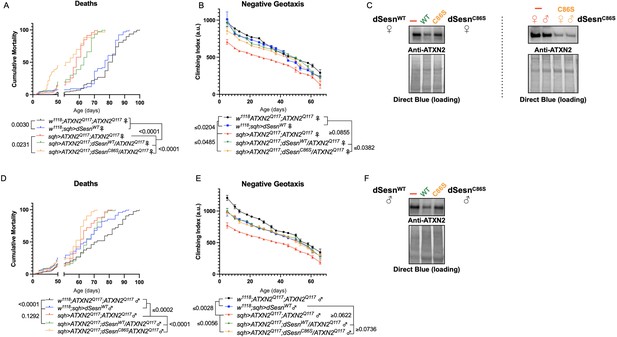
Oxidoreductase function is dispensable for mobility-extending effects of dSesn in flies expressing polyQ-expanded ATXN2.
Ubiquitous expression of dSesn harboring a point mutation that abolishes oxidoreductase activity (dSesnC86S, orange lines) exacerbates early death in SCA2 model flies (compare orange lines to red lines) in both females (A) and males (D). In contrast, climbing speed is partially rescued in the same female flies (expressing both dSesnC86S and polyQ-expanded ATXN2, in orange) (B) and fully rescued in males (E). (C, F) Both wild-type dSesn and dSesnC86S expression reduce ATXN2 levels, but the effect of dSesnC86S expression is variable (examples of variability shown in panel [C]). Survival and mobility experiments performed in triplicate. Individual survival experiments are composed of ≥200 flies/genotype, scored every second day for death and analyzed by log-rank tests. Each individual climbing datum depicts average climbing speed for 5 vials of 20 flies, analyzed by 2-way ANOVA. Error bars indicate ± SD. Representative Western blots from five biological replicates (five flies per lysate), quantified in Figure 7.
-
Figure 5—source data 1
Uncropped, unedited blots from Figure 5C (females) and Figure 5F (males).
- https://cdn.elifesciences.org/articles/75389/elife-75389-fig5-data1-v3.zip
-
Figure 5—source data 2
Uncropped, unedited blots from Figure 5C depicting examples of variabliity.
- https://cdn.elifesciences.org/articles/75389/elife-75389-fig5-data2-v3.zip
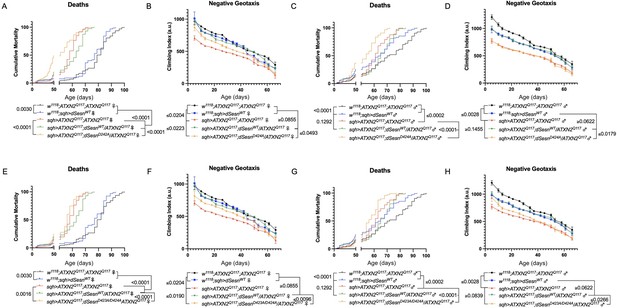
Interaction with mTOR is required for dSesn to improve survival and mobility in flies expressing polyQ-expanded ATXN2.
Ubiquitous expression of dSesn harboring two separate mutations that abolish mTORC interaction dSesnD424A (A–D), dSesn D423A/D424A (E–H) (denoted by orange lines) exacerbates early death (A,E, females, C,G, males) and fails to rescue mobility (B,F, females, D,H, males) in SCA2 flies. Survival and mobility experiments performed in triplicate. Individual survival experiments are composed of ≥200 flies/genotype, scored every second day for death and analyzed by log-rank tests. Each individual climbing datum depicts average climbing speed for ≥5 vials of 20 flies, analyzed by 2-way ANOVA. Error bars indicate ± SD.
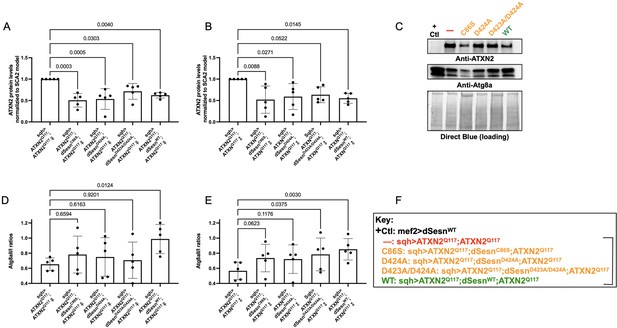
Wild-type dSesn expression reduces disease protein and increases autophagy in flies expressing polyQ-expanded ATXN2.
Ubiquitous expression of dSesn significantly reduces ATXN2 levels (A–C) and increases AtgIIa;AtgIa ratios (C–E). n = 5 biological replicates, 5 flies per lysate, analyzed by ANOVA with Tukey post-hoc comparison for significantly different groups. (F) Genotypes in representative Western blot and from independent biological replicates, quantified in (A, B, D, E). Bracket in (F) indicates quantifications in (A, B, D, E).
-
Figure 7—source data 1
Uncropped, unedited blots from Figure 7C.
- https://cdn.elifesciences.org/articles/75389/elife-75389-fig7-data1-v3.zip
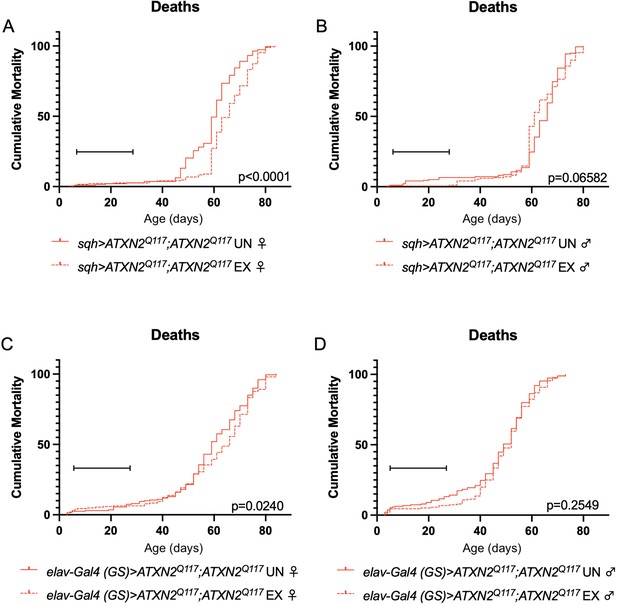
Exercise reduces early death in SCA2 model flies expressing two copies of ATXN2.
Exercised female flies expressing two copies of CAG-expanded ATXN2 ubiquitously (A) or in adult neurons (C) have increased survival compared to age-matched, unexercised siblings, while exercised male flies expressing two copies of CAG-expanded ATXN2 ubiquitously (B) or in adult neurons (D) trend toward increased survival only in the first 25 days, the period in which flies are still training. Brackets indicate exercise training period. p-Values indicate log-rank for entire survival curve, n ≥ 170, performed in duplicate.
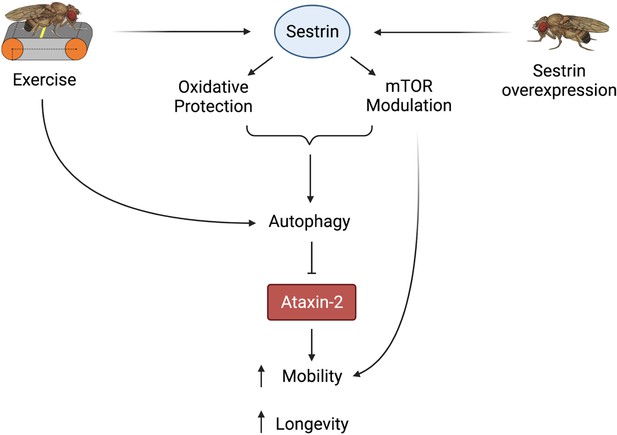
Proposed model of the effects of exercise and dSesn overexpression on SCA2 flies.
Sesn’s known functions and activation by exercise have been established previously (Kim et al., 2020; Kim et al., 2015). Wild-type Sesn overexpression or exercise can activate autophagy and reduce disease protein levels, improving function. Source data-all figures. Tab delimited excel file containing raw data for all figures and figure supplements. Uncropped, unedited Western blot data are included as separate images.
Videos
SCA2 model flies show improved motility with exercise.
Video of 4-week-old uninduced control and SCA2 model flies taken after 3 weeks of ramped endurance exercise. Order of vials depicted, left to right: Vial 1-uninduced, exercised control, Vials 2,3-SCA2 model, exercised, Vials 4,5-SCA2 model, unexercised.
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Gene (H. sapiens) | ATXN2 | GenBank | FLYB: FBgn0267931 | |
| Gene (H. sapiens) | ATXN3 | GenBank | FLYB: FBgn0024961 | |
| Gene (H. sapiens) | CACNA1A | GenBank | FLYB: FBgn0283733 | |
| Gene (D. melanogaster) | Sesn | GenBank | FLYB: FBgn0034897 | |
| Genetic reagent (D. melanogaster) | UAS- ATXN2Q117 | Bloomington Drosophila Stock Center | BDSC68394: FBst0068394 | Flybase Symbol:w*; P{UAS-ATXN2.117Q}8B |
| Genetic reagent (D. melanogaster) | UAS- ATXN2Q117 | Bloomington Drosophila Stock Center | BDSC68395: FBst0068395 | Flybase Symbol: w*; P{UAS-ATXN2.117Q}9 A |
| Genetic reagent (D. melanogaster) | UAS-ATXN3Q77 | Tsou et al., 2016 | ||
| Genetic reagent (D. melanogaster) | UAS-α1ACTQ70 | Sutton et al., 2017 | ||
| Genetic reagent (D. melanogaster) | w1118;UAS-ATXN2Q117/CyO;UAS-ATXN2Q117 | This paper | See methods lines 305–306 | |
| Genetic reagent (D. melanogaster) | w1118;sqh/CyO;UAS- dSesnWT/TM3-Sb | This paper | See methods lines 311–314 | |
| Genetic reagent (D. melanogaster) | w1118;sqh/CyO;UAS- dSesnC86S/TM3-Sb | This paper | See methods lines 311–314 | |
| Genetic reagent (D. melanogaster) | w1118;sqh/CyO;UAS- dSesnD424A/TM3-Sb | This paper | See methods lines 311–314 | |
| Genetic reagent (D. melanogaster) | w1118;sqh/CyO;UAS- dSesnD423A/D424A/TM3-Sb | This paper | See methods lines 311–314 | |
| Antibody | anti-ataxin-2 (mouse monoclonal) | BD biosciences | 611,378 | (1:500) |
| Antibody | anti-ataxin-3 (mouse monoclonal) | Millipore | 1H9, MAB5360 | (1:1000) |
| Antibody | anti-GABARAP (rabbit polyclonal) | Abcam | Ab1398 | (1:1000) |
| Antibody | anti-dSesn (rabbit polyclonal) | Kim et al., 2020 | (1:500) |
Additional files
-
Transparent reporting form
- https://cdn.elifesciences.org/articles/75389/elife-75389-transrepform1-v3.docx
-
Source data 1
- https://cdn.elifesciences.org/articles/75389/elife-75389-data1-v3.zip





