Pigment cell progenitor heterogeneity and reiteration of developmental signaling underlie melanocyte regeneration in zebrafish
Figures
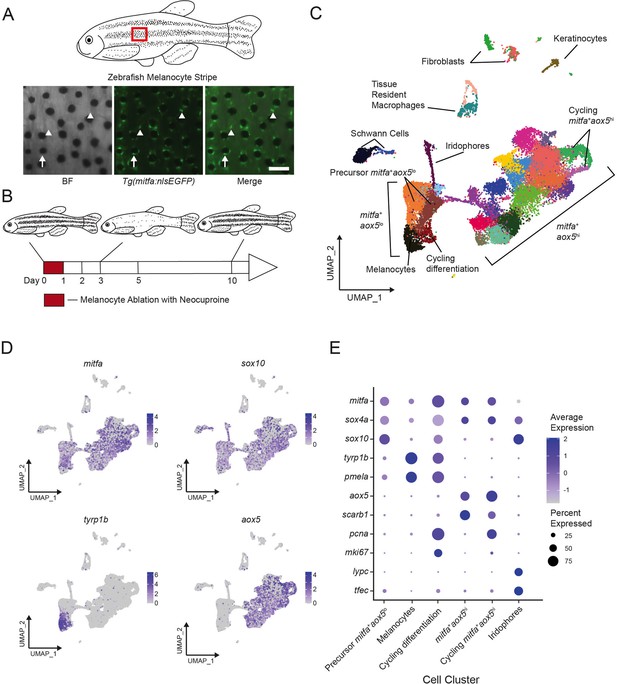
Single-cell transcriptomic identification of melanocyte lineage cells during regeneration.
(A) Top, diagram of zebrafish flank with melanocyte stripes. Bottom, representative images of the melanocyte stripe in a Tg(mitfa:nlsEGFP) zebrafish. Melanocyte progenitors are unpigmented GFP-expressing cells (arrowheads) admixed with pigmented melanocytes (arrows). Animals were treated with epinephrine prior to imaging to concentrate melanosomes into the cell body of melanocytes. Scale bar = 100 µm. (B) Experimental design for transcriptional profiling of progenitors in Tg(mitfa:nlsEGFP) zebrafish during melanocyte ablation and regeneration. Cells from Tg(mitfa:nlsEGFP) zebrafish were sampled at the days specified. (C) UMAP of cell-type assignments for clusters of cells obtained from Tg(mitfa:nls:EGFP) zebrafish. Cells from all time points were included. Coloring is according to unsupervised clustering (Blondel et al., 2008; Stuart et al., 2019). Cells are labeled based on gene expression patterns revealed in panels (D) and (E) and Figure 1—figure supplement 2A. The large group of cells to the bottom left in the UMAP are mitfa+aox5lo. Within this group of cells, the mitfa+aox5lotyrp1b+pcna− cells are designated as melanocytes, the mitfa+aox5lotyrp1b+pcna+ are designated ‘cycling differentiation’, and the other two populations are designated ‘precursor mitfa+aox5lo’. The larger group of cells to the bottom right in the UMAP are mitfa+aox5hi. Within this group of cells two clusters are pcna+ are designated ‘cycling mitfa+aox5hi’ (n = 29,453 cells). (D) Expression of pigment cell markers mitfa and aox5, melanin biosynthesis gene tyrp1b and pigment cell progenitor marker sox10 shown as feature plots on the UMAP plot from (C). (E) Expression of pigment cell markers, the stem cell gene sox4a, and cell cycle markers for cell clusters shown in panel C. Dot sizes represent percentage of cells in the cluster expressing the marker and coloring represents average expression.
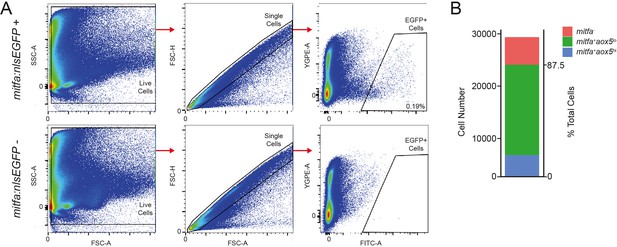
Isolation strategy and characteristics of mitfa:nlsEGFP-expressing cells.
(A) Representative flourescence-activated cell sorting (FACS) plots and gating strategy to isolate mitfa:nlsEGFP-positive cells from zebrafish skin. Top, isolation from skin of Tg(mitfa:nlsEGFP) zebrafish, and, bottom, from negative control skin of wild-type zebrafish. The percentage of mitfa:nlsEGFP-positive cells (0.19%) is expressed as the proportion of cells that are gated as singlets (middle panel) and EGFP-positive gate (right panel) as compared to the total number of cells in the live cell gate (left panel). (B) Percentage of mitfa-expressing cells from FACS isolation, based on scRNAseq analyses (n = 29,453 cells).
-
Figure 1—figure supplement 1—source data 1
Cell number and percentage of mitfa-expressing cells from FACS isolation, based on scRNAseq analyses.
- https://cdn.elifesciences.org/articles/78942/elife-78942-fig1-figsupp1-data1-v2.xlsx
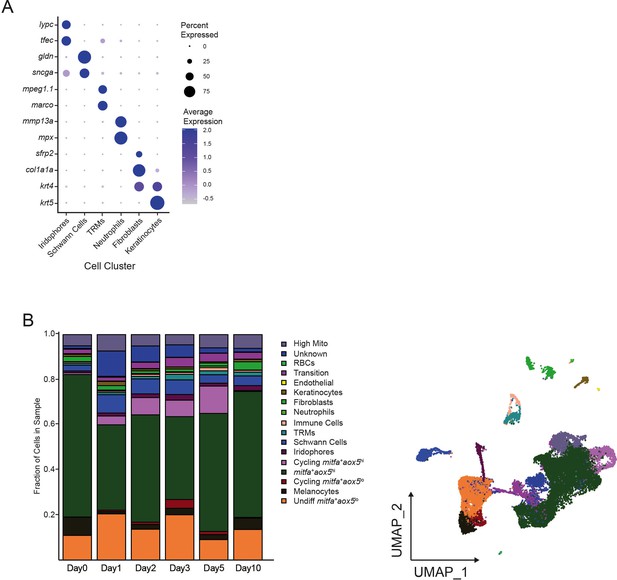
Isolation strategy and characteristics of mitfa:nlsEGFP-expressing cells.
(A) Expression of cell markers for cell clusters shown in Figure 1A. Dot sizes represent percentage of cells in the cluster expressing the marker and coloring represents average expression. (B) Left, proportions of cell types across all Tg(mitfa:nlsEGFP) samples. High Mito: high mitochondrial read fraction cells; Unknown: cells with unclear literature-supported markers; RBCs: red blood cells; Transition: cells spanning a geographic transition between the mitfa-expression populations; TRMs: tissue-resident macrophages (Day 0 = 7989, Day 1 = 4004, Day 2 = 4510, Day 3 = 5224, Day 5 = 3483, Day 10 = 4243 total cells per sample). Right, corresponding UMAP of broad cell-type assignments based on cell markers shown in Figure 1E and (A).
-
Figure 1—figure supplement 2—source data 1
Proportions of cell types across all Tg(mitfa:nlsEGFP) samples.
- https://cdn.elifesciences.org/articles/78942/elife-78942-fig1-figsupp2-data1-v2.xlsx
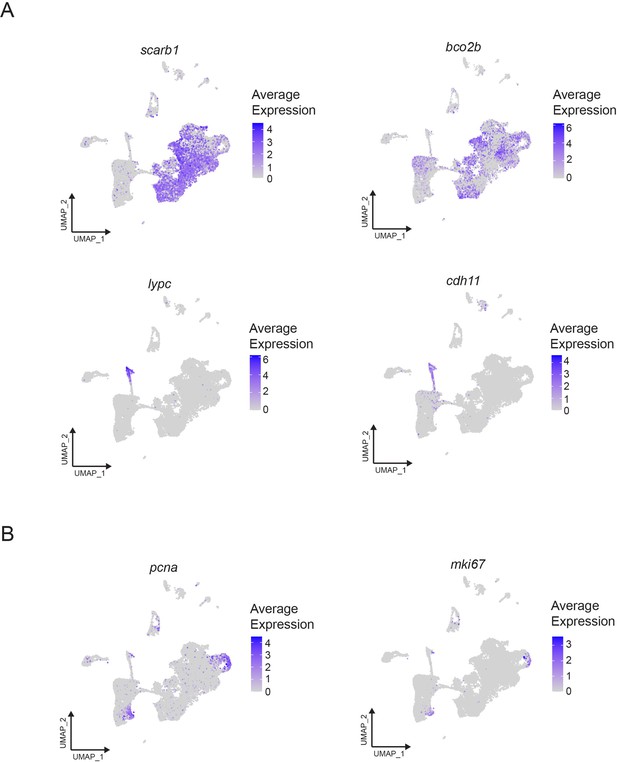
Expression of xanthophore, iridophore, and cell cycle markers.
(A) Top, expression of scarb1 and bco2b, which are expressed in differentiated xanthophores and pigment cell progenitors. Bottom, expression of lypc and cdh11, which are expressed in differentiated iridophores. (B) Expression of cell cycle markers pcna, mki67. Expression of all genes is shown as feature plots on the UMAP plot from Figure 1C.
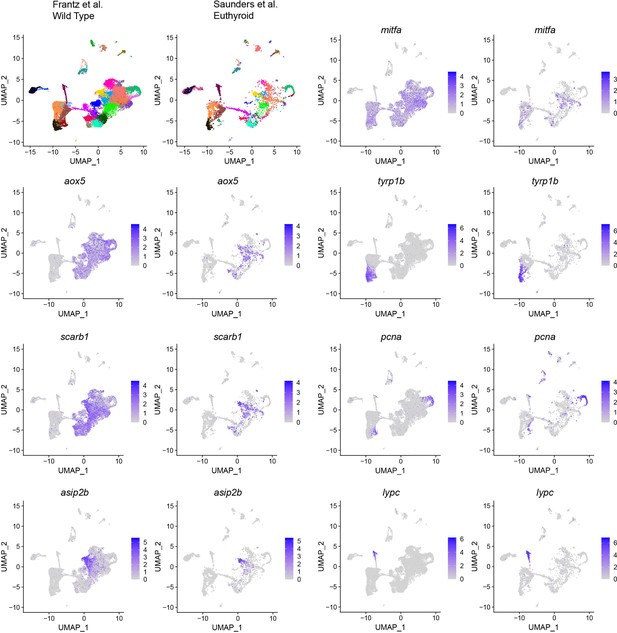
Comparison of this article's and Saunders et al., 2019 zebrafish pigment cell lineage datasets with expression of melanocyte, xanthophore, iridophore, and cell cycle markers.
Euthyroid cells from Saunders et al., 2019 mapped onto clusters from Figure 1C using WT cells as a reference (top left). Expression of melanocyte cell markers tyrp1b and mitfa, xanthophore pigment cell markers aox5, scarb1, asip2b, iridophore marker gene lypc, and cell cycle marker pcna shown as feature plots on the UMAP in the top left.
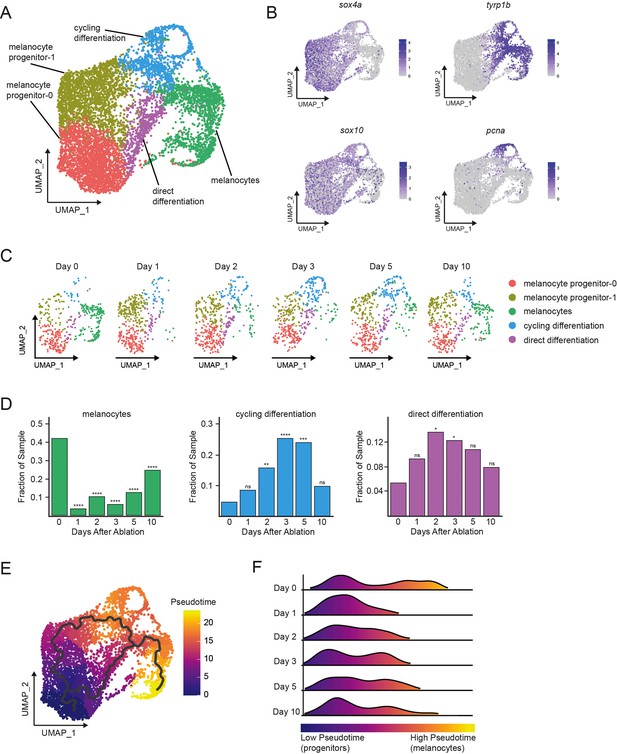
Single-cell transcriptomics reveal dynamic cell and gene expression changes following melanocyte ablation.
(A) Integrated subclustering of WT mitfa+aox5lo cells before, during, and after melanocyte regeneration (n = 5619 cells). Coloring is according to unsupervised clustering (Blondel et al., 2008; Stuart et al., 2019). Cell clusters are labeled based on gene expression patterns and inferred trajectories during regeneration, as revealed in panels (B–F) and Figure 2—figure supplement 1. Melanocyte and cycling differentiation clusters are the same as in Figure 1A, whereas the two precursor mitfa+aox5lo clusters from Figure 1A are now resolved into three clusters: melanocyte progenitor-0 (MP-0), melanocyte progenitor-1, and ‘direct differentiation’, the latter of which expresses the stem cell marker sox4a, the melanin biosynthesis gene tyrp1b and is pcna negative. (B) Expression of stem cell marker sox4a, melanin biosynthesis gene tyrp1b, pigment cell progenitor marker sox10, and cell cycle gene pcna as feature plots on the UMAP plot from (A). (C) Dynamic changes in subpopulations of WT mitfa+aox5lo cells. Biological sample runs were downsampled to a common number of total cells so shifts in cluster proportions could be readily visualized. (D) Quantification of proportion of cells per scRNAseq sample, comparing the indicated time point to day 0, in the melanocyte, cycling differentiation, and direct differentiation subpopulations during regeneration in WT animals (Day 0 = 7989, Day 1 = 4004, Day 2 = 4510, Day 3 = 5224, Day 5 = 3483, Day 10 = 4243 total cells per sample). p values calculated using differential proportion analysis (Farbehi et al., 2019), *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001; ns, not significant. (E) Cellular trajectories, as determined by Monocle3 (Cao et al., 2019) projected onto the mitfa+aox5lo subcluster (left panel). Solid lines represent trajectories, with an origin in the MP-0 subpopulation and two distinct paths through different intermediate cell subpopulations. (F) These trajectories are then used to calculate pseudotime, which, as an approximation of biological time, reveals how low pseudotime progenitors (blue) progress across transitional cell types to high pseudotime melanocytes (yellow). Ridge plot of the distribution of pseudotime during regeneration. Height of ridge corresponds to number of cells at that pseudotime.
-
Figure 2—source data 1
Proportion of cells per scRNAseq sample in the melanocyte, cycling differentiation, and direct differentiation subpopulations.
- https://cdn.elifesciences.org/articles/78942/elife-78942-fig2-data1-v2.xlsx
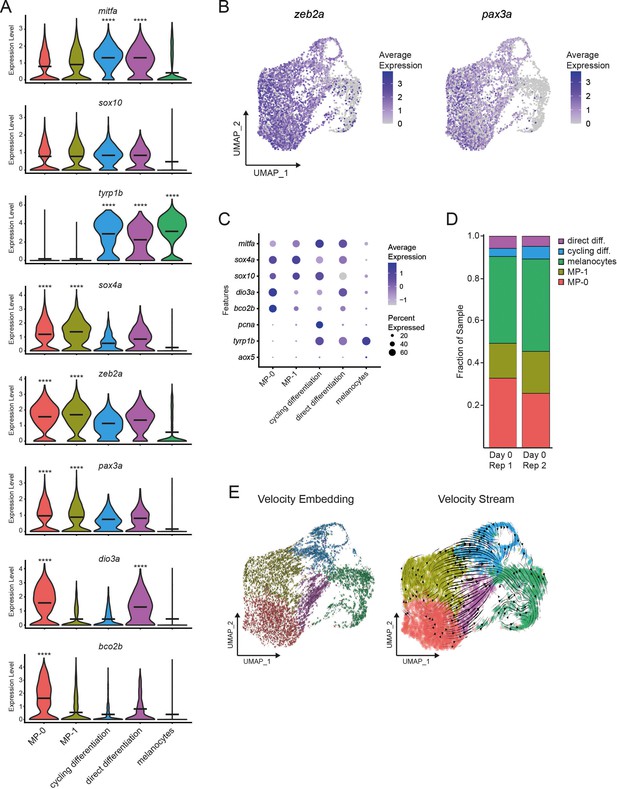
Single-cell profiling supports progenitor identity and subpopulation dynamics.
(A) Violin plots of cell subpopulation expression of mitfa, pigment cell progenitor (sox10), melanin biosynthesis (tyrp1b), stem cell (sox4a), and neural crest (zeb2a, pax3a) genes. Genes distinguishing the two progenitor clusters (dio3a, bco2b) are also visualized (MP-0 = 1840, MP-1 = 1408, cycling differentiation = 777, direct differentiation = 535 , and melanocytes = 1060 cells). Black bars represent mean gene expression. p values calculated by Wilcoxon rank-sum test, ****p < 0.0001. (B) Expression of neural crest genes zeb2a and pax3a and their enrichment in melanocyte progenitor-0 (MP-0) and melanocyte progenitor-1 (MP-1) subpopulations as feature plots on the UMAP plot from Figure 2A. (C) Dot plot demonstrating gene expression differences between mitfa+aox5lo cell subpopulations. Dot sizes represent percentage of cells in the cluster expressing the marker and coloring represents average expression. (D) Comparison of mitfa+aox5lo cell subpopulation proportions between different scRNAseq samples of unperturbed (day 0) wild-type zebrafish skin (Rep 1 = 5269 and Rep 2 = 2720 cells per sample). (E) RNA splicing mechanics scored using velocity embedding (velocyto; La Manno et al., 2018) and visualized through velocity stream analysis (scvelo; Bergen et al., 2020) on the UMAP plot from Figure 2A.
-
Figure 2—figure supplement 1—source data 1
Comparison of mitfa+aox5lo cell subpopulation proportions between scRNAseq samples of unperturbed (day 0) wild-type zebrafish skin.
- https://cdn.elifesciences.org/articles/78942/elife-78942-fig2-figsupp1-data1-v2.xlsx
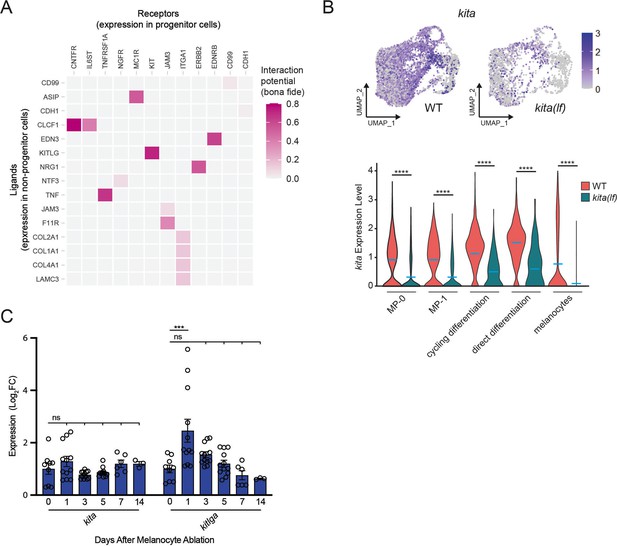
NicheNet and transcriptional analyses implicate the KIT signaling axis as a dynamic regulator of melanocyte regeneration.
(A) Heatmap of NicheNetR-identified ligand/receptor pairs indicating interaction potential between receptors on progenitor receiver cells and ligands on non-progenitor sender cells sampled by scRNAseq. Ligand/receptor pairs were restricted to literature-supported pairs (Browaeys et al., 2020). (B) Top, feature and, bottom, violin plots of kita expression in mitfa+aox5lo cells from wild-type (MP-0 = 1840, MP-1 = 1408, cycling differentiation = 777, direct differentiation = 535, and melanocytes = 1060 cells) and kita(lf) strains (MP-0 = 617, MP-1 = 612, cycling differentiation = 280, direct differentiation = 57, and melanocytes = 563 cells). Mean gene expression represented by cyan bars. p values calculated by Wilcoxon rank-sum test, ****p < 0.0001. (C) Quantitative real-time polymerase chain reaction (qRT-PCR) of kita and kitlga expression in zebrafish skin following melanocyte destruction. Three biological replicates were performed for each time point. Data are shown as mean ± standard error of the mean (SEM). p values calculated by one-way analysis of variance (ANOVA) with Dunnett’s multiple comparisons test, ***p < 0.001; ns, not significant.
-
Figure 3—source data 1
qRT-PCR of kita and kitlga expression in zebrafish skin following melanocyte destruction.
- https://cdn.elifesciences.org/articles/78942/elife-78942-fig3-data1-v2.xlsx
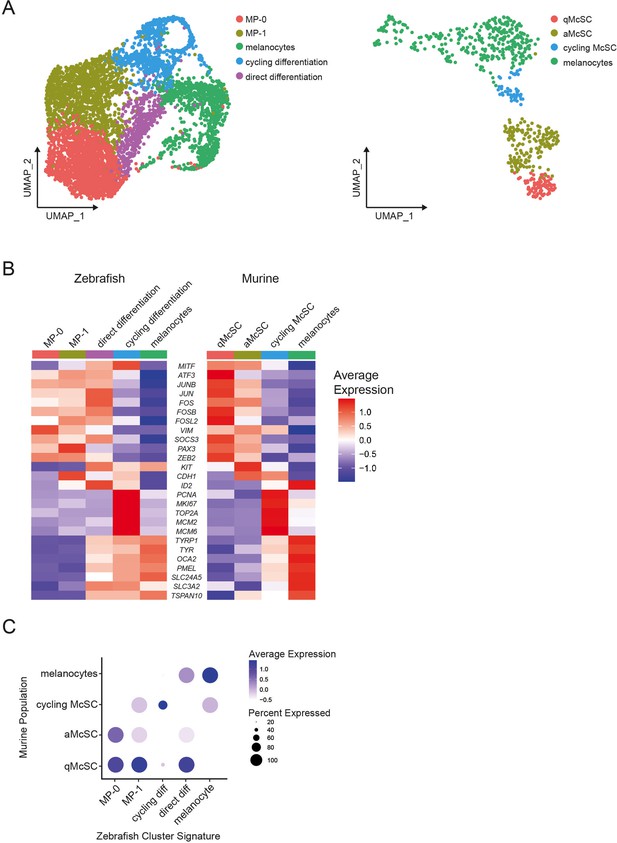
Zebrafish progenitor regenerative signature is conserved across species.
(A) Left, UMAP subclustering of mitfa+aox5lo cells before, during, and after melanocyte regeneration as in Figure 2A (n = 5619 cells). Right, integrated subclustering of murine melanocyte stem cells (McSCs), intermediate cell populations and melanocytes during hair cycle turnover (n = 626 cells); qMcSC, quiescent McSCs; aMcSC, activated McSCs (Infarinato et al., 2020). (B) Heatmaps of melanocyte lineage cluster markers conserved across zebrafish and murine scRNAseq datasets. Gene labels are human orthologs of genes expressed as cluster markers in both zebrafish (left) and murine (right). Gene expression is row normalized. (C) Dot plot visualizing conserved regenerative transcriptional states across species. Zebrafish subpopulation signatures were calculated using the FindMarkers feature in Seurat (Satija et al., 2015; Stuart et al., 2019) from zebrafish subpopulations then the top 100 differentially expressed genes (DEGs) from each zebrafish signatures were plotted onto murine subpopulations (Infarinato et al., 2020). Dot sizes represent percentage of cells in the murine subpopulation expressing the zebrafish gene signature and coloring represents average expression.
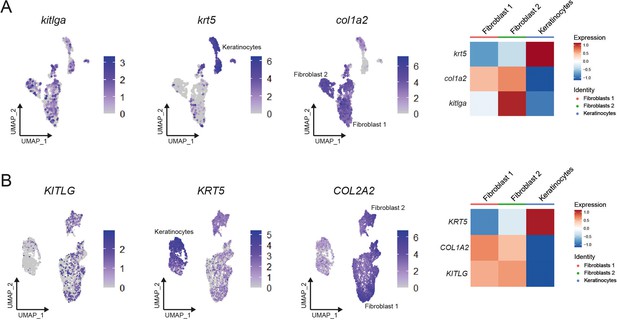
kitlga ligand expression in zebrafish keratinocytes and fibroblasts is similar to that observed in human cells.
(A) Left, feature plots of zebrafish kitlga expression, keratinocyte marker krt5, and fibroblast marker col1a2. Right, heatmap plots of kitlga, krt5, and col1a2 expression in keratinocytes and fibroblasts. (B) Left, feature plots of human KITLG expression, keratinocyte marker KRT5, and fibroblast marker COL2A2 (Joost et al., 2020). Right, heatmap plots of KITLG, KRT5, and COL2A2 expression in keratinocytes and fibroblasts.
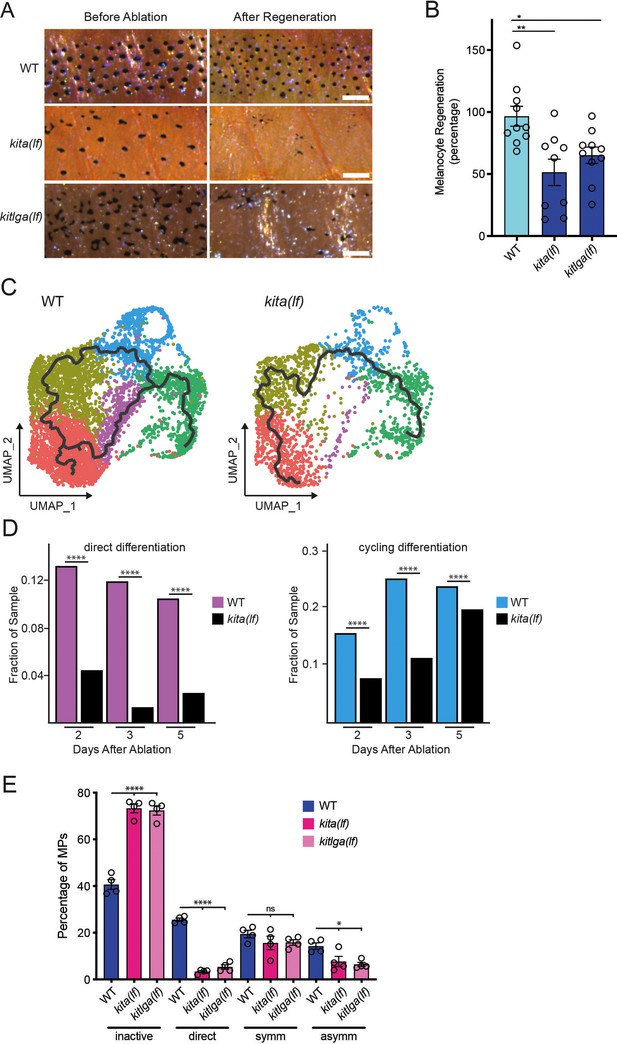
kita receptor and kitlga ligand loss-of-function mutants have impaired melanocyte regeneration.
(A) Brightfield images of the melanocyte stripe before melanocyte ablation and after melanocyte regeneration in wild-type, kita(lf), and kitlga(lf) zebrafish strains. Scale bar = 200 µm. (B) Quantification of melanocyte regeneration in wild-type, kita(lf), and kitlga(lf) strains. Mean percentage ± standard error of the mean (SEM) is shown; WT n = 10, kita(lf) = 9, kitlga(lf) = 11 fish. p values calculated by one-way analysis of variance (ANOVA) with Dunnett’s multiple comparisons test, *p < 0.05, **p < 0.01. (C) Cellular trajectories, using Monocle3, of mitfa+aox5lo cells from wild-type zebrafish (left) and kita(lf) mutants (right). Solid lines represent trajectories, with an origin in the melanocyte progenitor-0 (MP-0) subpopulation and terminus in the melanocyte subpopulation. (D) Comparison of proportion of WT and kita(lf) cells per scRNAseq sample in the cycling differentiation and direct differentiation subpopulations during regeneration reveals fewer kita(lf) cells going through differentiation (WT Day 2 = 4510, WT Day 3 = 5224, WT Day 5 = 3483, kita(lf) Day 2 = 4233, kita(lf) Day 3 = 5261, kita(lf) Day 5 = 5411 total cells per sample). p values calculated using differential proportion analysis (Farbehi et al., 2019), ****p < 0.0001; ns, not significant. (E) Fates of progenitors following single-cell serial imaging of wild-type, kita(lf), and kitlga(lf) strains. Mean percentage of traced progenitors in a fate are shown; wild-type n = 4 animals (n = 49, 59, 53, 53 cells per animal), kita(lf) = 4 animals (n = 50, 50, 53, 28 cells per animal), kitlga(lf) = 4 animals (n = 52, 39, 53, 33 cells per animal). p values calculated by one-way ANOVA with Dunnett’s multiple comparisons test, ****p < 0.0001, *p < 0.05, ns, not significant.
-
Figure 4—source data 1
KIT signaling mutants have impaired melanocyte regeneration.
(B) Percentage of melanocyte regeneration in wild-type, kita(lf), and kitlga(lf) strains. (D) Proportion of WT and kita(lf) cells per scRNAseq sample in the cycling differentiation and direct differentiation subpopulations during regeneration. (E) Fates of progenitors following single-cell serial imaging of wild-type, kita(lf), and kitlga(lf) strains.
- https://cdn.elifesciences.org/articles/78942/elife-78942-fig4-data1-v2.xlsx
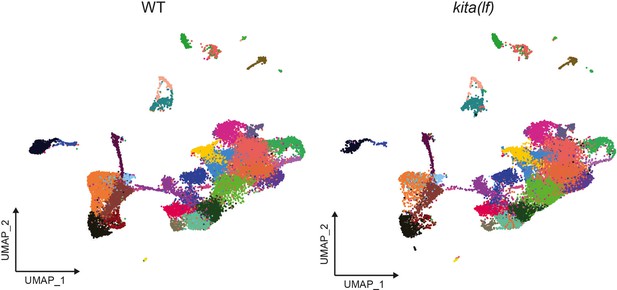
kita(lf) animals demonstrate conservation of cell types found in WT animals (clustered together).
Unsupervised clustering of all integrated wild-type (left, n = 29,453 cells) and kita(lf) (right, n = 24,724 cells) cells profiled by scRNAseq.
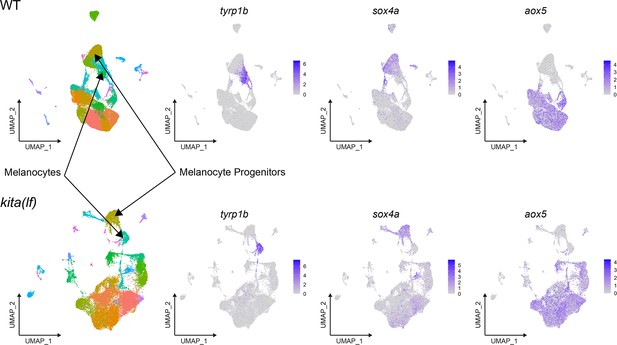
kita(lf) animals demonstrate conservation of cell types found in WT animals (clustered separately).
Unsupervised clustering of wild-type cells (top left, n = 29,453 cells) and separate unsupervised clustering of kita(lf) cells (bottom left, n = 24,724 cells) profiled by scRNAseq. Expression of melanin synthesis gene tyrp1b, stem cell marker sox4a, and pteridine processing gene aox5.
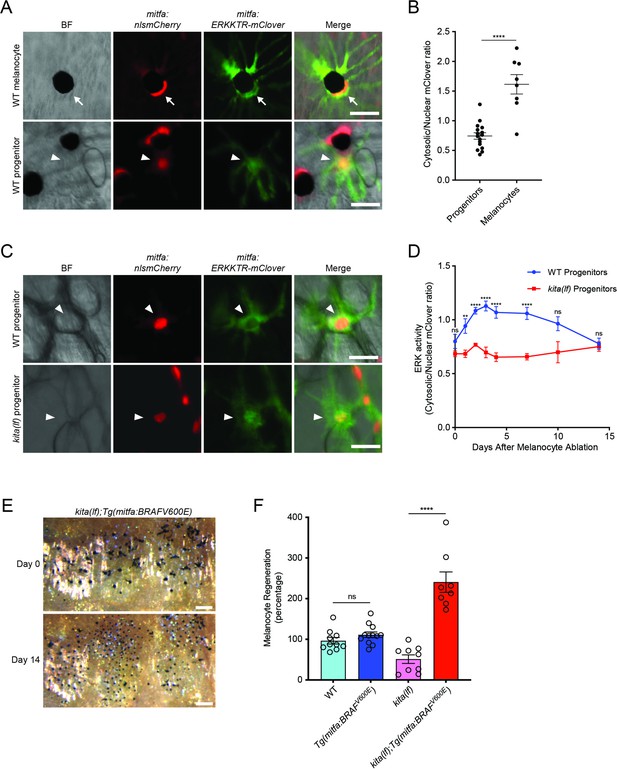
kitlga/kita signaling during melanocyte regeneration acts through the MAPK pathway.
(A) Images of ERKKTR-mClover localization in a representative mature melanocyte (top, arrow) and progenitor (bottom, arrowhead) in uninjured wild-type animals. Scale bar = 30 µm. (B) Quantification of ERK activity in progenitors and melanocytes based on ERKKTR-mClover localization. Mean ± standard error of the mean (SEM) is shown; progenitors n = 16, melanocytes n = 8. (C) Images 3 days post-ablation of ERKKTR-mClover location in representative progenitors (arrowheads) in wild-type (top) and kita(lf) (bottom) animals. Scale bar = 30 µm. (D) Quantification of ERK activity in progenitors prior to and during melanocyte regeneration. For each data point, the average cytosolic/nuclear ratio of at least 6 cells ± SEM is shown. (E) Brightfield images of the melanocyte stripe before (top) and after (bottom) regeneration in kita(lf); Tg(mitfa:BRAFV600E) mutants. Scale bar = 200 µm. (F) Quantification of melanocyte regeneration in kita(lf);Tg(mitfa:BRAFV600E) and control animals. Mean percentage ± SEM is shown; wild-type n = 10, Tg(mitfa:BRAFV600E) = 12, kita(lf) = 9, kita(lf);Tg(mitfa:BRAFV600E) = 8 fish. p values calculated by Student’s t-test, **p < 0.01, ****p < 0.0001; ns, not significant.
-
Figure 5—source data 1
KIT signaling during melanocyte regeneration acts through the MAPK pathway.
(B) Quantification of ERK activity in progenitors and melanocytes. (D) Quantification of ERK activity in progenitors prior to and during melanocyte regeneration. (F) Quantification of melanocyte regeneration in kita(lf);Tg(mitfa:BRAFV600E) and control animals.
- https://cdn.elifesciences.org/articles/78942/elife-78942-fig5-data1-v2.xlsx
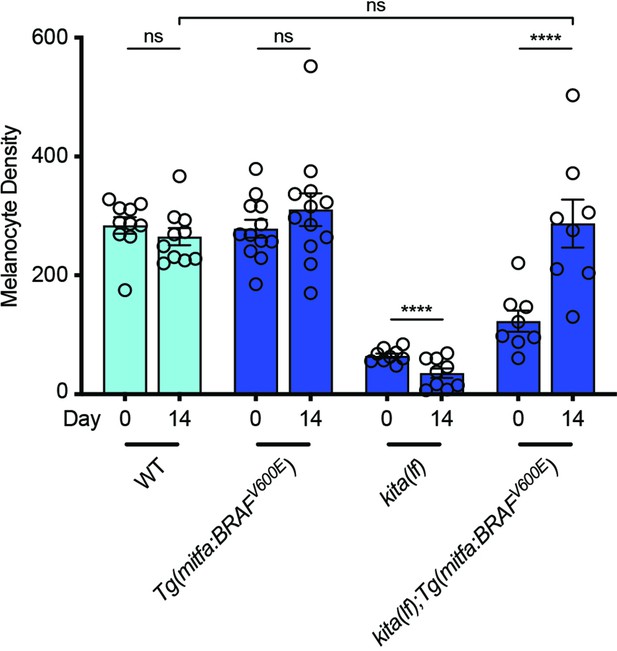
kita(lf) animals with constitutively active BRAF regenerate a similar number of melanocytes as wild-type animals.
Mean number of melanocytes from the middle stripe per field ± standard error of the mean (SEM) is shown; wild-type n = 10, Tg(mitfa:BRAFV600E) = 12, kita(lf) = 9, kita(lf); Tg(mitfa:BRAFV600E) = 8 fish. p values calculated by Student’s t-test, ****p < 0.0001; ns, not significant.
-
Figure 5—figure supplement 1—source data 1
Number of melanocytes from the middle stripe per field in wild-type, Tg(mitfa:BRAFV600E), kita(lf), and kita(lf); Tg(mitfa:BRAFV600E) animals.
- https://cdn.elifesciences.org/articles/78942/elife-78942-fig5-figsupp1-data1-v2.xlsx
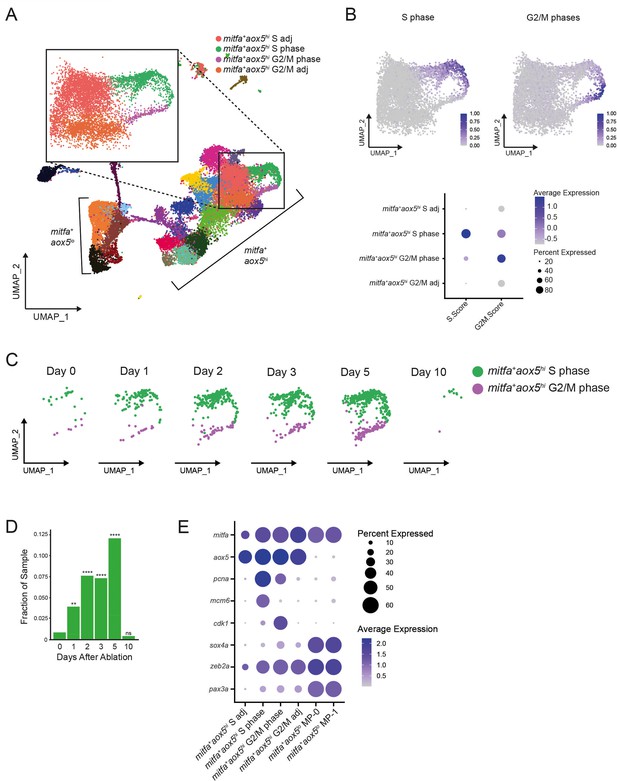
A subpopulation of mitfa+aox5hi cells divides and expands during regeneration.
(A) UMAP of all cells sampled from wild-type animals with enlargement of cycling and adjacent subpopulations found in the large group of mitfa+aox5hi cells. (B) Top, feature and, bottom, dot plot of S phase and G2/M phase cell cycle scores of cells highlighted in (A). Cell cycle scores were calculated using Seurat’s ‘CellCycleScoring’ module and zebrafish orthologs of the cell cycle genes outlined by Tirosh et al., 2016. (C) UMAPs of cycling cells prior to and during regeneration. Biological sample runs were downsampled to a common number of total cells so shifts in clusters could be readily visualized. Cells in S phase are seen in green, and cells in G2/M phase are seen in purple. (D) Quantification of proportion of cells per scRNAseq sample in the mitfa+aox5hi cycling subpopulations (mitfa+aox5hi S phase and mitfa+aox5hi G2/M subpopulations combined) during regeneration (Day 0 = 7989, Day 1 = 4004, Day 2 = 4510, Day 3 = 5224, Day 5 = 3483, Day 10 = 4243 total cells per sample). p values calculated using differential proportion analysis (Farbehi et al., 2019), **p < 0.01; ****p < 0.0001; ns, not significant. (E) Dot plot of pigment cell and cell cycle marker genes differentially expressed across S adj, cycling, and G2/M adj mitfa+aox5hi subpopulations during melanocyte regeneration. Dot sizes represent percentage of cells in the cluster expressing the marker and coloring represents average expression.
-
Figure 6—source data 1
Proportion of cells per scRNAseq sample in the mitfa+aox5hi cycling subpopulations during regeneration.
- https://cdn.elifesciences.org/articles/78942/elife-78942-fig6-data1-v2.xlsx
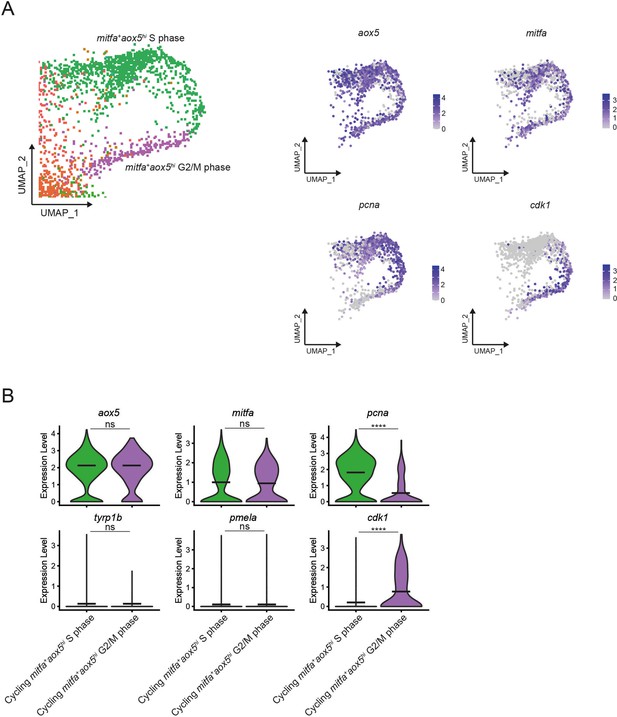
Gene expression in mitfa+aox5hi cycling subpopulations.
(A) Left, enlargement of the mitfa+aox5hi cycling subpopulation from Figure 6A showing two clusters. Right, feature plots of expression of pigment cell markers and cell cycle genes in the mitfa+aox5hi cycling clusters. (B) Violin plots of gene expression of pigment cell markers and cell cycle genes of the clusters seen in Figure 6A and (A) (S phase = 1136 and G2/M phase = 254 cells). Black bars represent mean gene expression. p values calculated by Wilcoxon rank-sum test, ****p < 0.0001; ns, not significant.
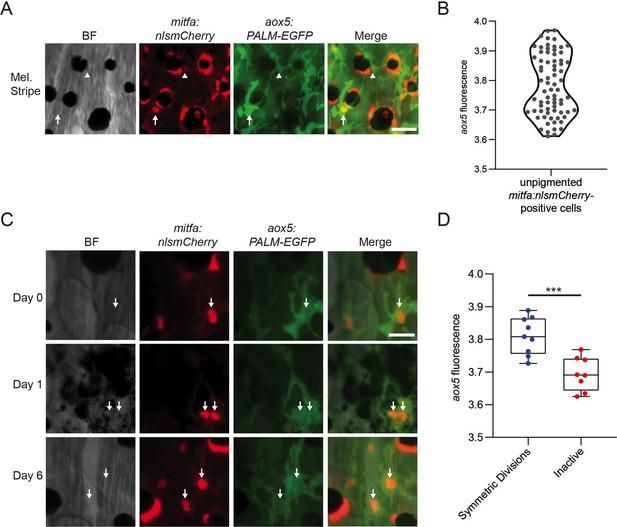
aox5 expression predicts in vivo progenitor cell fate.
(A) Representative image of progenitors expressing different levels of aox5 promoter-driven PALM-EGFP. mitfa+aox5hi (arrowhead), mitfa+aox5lo (arrow). Scale bar = 50 µm. (B) Quantification of PALM-EGFP fluorescence intensity indicates groups of progenitors that express lower and higher levels of aox5. Mean pixel intensity per area; intensity values log normalized. n = 73 cells from 5 animals. (C) Images of an mitfa+aox5hi cell that underwent mitosis following melanocyte destruction. Scale bar = 30 µm. (D) Comparison of aox5 intensity in cells that underwent symmetric divisions or remained inactive. Mean pixel intensity per area; intensity values log normalized. n = 17 cells from 5 animals. p values calculated by Student’s t-test, ***p < 0.001.
-
Figure 7—source data 1
aox5 expression in progenitors.
(B) PALM-EGFP fluorescence intensity of progenitors. (D) aox5 intensity in cells that underwent symmetric divisions or remained inactive.
- https://cdn.elifesciences.org/articles/78942/elife-78942-fig7-data1-v2.xlsx
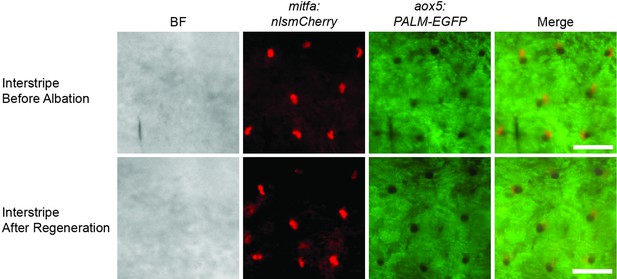
Lack of interstripe xanthophore divisions following neocuproine treatment.
Representative images from serial imaging of interstripe xanthophores from Tg(mitfa:nlsmCherry); Tg(aox5:PALM-EGFP) animals following neocuproine treatment. Scale bar = 60 µm.
Videos
Serial imaging of direct differentiation.
Representative images from serial imaging of an unpigmented mitfa-expressing cell undergoing direct differentiation in the melanocyte stripe of a Tg(mitfa:nlsEGFP) zebrafish. Merged brightfield and GFP. White arrowheads indicate traced cell. Scale bar = 50 µm.
Serial imaging of symmetric division.
Representative images from serial imaging of an unpigmented mitfa-expressing cell undergoing symmetric division in the melanocyte stripe of a Tg(mitfa:nlsEGFP) zebrafish. Merged brightfield and GFP. White arrowheads indicate traced cell and subsequent daughter cells. Scale bar = 50 µm.
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Chemical compound, drug | Neocuproine | Sigma-Aldrich | ID_source:N1501 | |
| Chemical compound, drug | (−)-Epinephrine (+)-bitartrate salt, 98+% | Acros Organics | ID_source:AC430140010 | |
| Chemical compound, drug | Tricaine methanesulfonate | Syndel | ||
| Chemical compound, drug | TM Liberase | Sigma-Aldrich | ID_source:LIBTM-RO | |
| Strain (Danio rerio) | WT (AB) | Gift. | ||
| Strain (Danio rerio) | kita(lf) | Gift. Parichy et al., 1999 PMID:10393121 | ||
| Strain (Danio rerio) | kitlga(lf) | Gift. Hultman et al., 2007 PMID:17257055 | ||
| Strain (Danio rerio) | Tg(mitfa:nlsEGFP) | This paper | Figure 1, Methods: DNA constructs, Transgenic Fish | |
| Strain (Danio rerio) | Tg(mitfa:nlsmCherry) | This paper | Figure 1, Methods: DNA constructs, Transgenic Fish | |
| Strain (Danio rerio) | Tg(mitfa:ERKKTR-mClover) | This paper, Mayr et al., 2018 PMID:30320107 | Figure 5, Methods: DNA constructs, Transgenic Fish | |
| Strain (Danio rerio) | Tg(aox5:PALM-EGFP) | Gift. Eom et al., 2015 PMID:26701906 | ||
| Gene (Danio rerio) | kitlga | Parichy et al., 1999 PMID:10393121 | Amplified from cDNA | |
| Gene (Danio rerio) | kita | Hultman et al., 2007 PMID:17257055 | Amplified from cDNA | |
| Commercial kit | SYBR Green | Thermo Fisher | ID_source:4472908 | |
| Commercial kit | SuperScript III First-Strand Synthesis System for RT-PCR | Invitrogen | ID_source:18080-051 | |
| Commercial kit | Chromium Next GEM Single Cell 3′ GEM, Library, and Gel Bead Kit v3.1 | 10× Genomics | ID_source:1000128 | |
| Software, algorithm | Cell Ranger | 10× Genomics | V3.0.0 | |
| Software, algorithm | Seurat | Stuart et al., 2019 PMID:31178118 | V3 | https://github.com/satijalab/seurat; satijalab, 2022 |
| Software, algorithm | Monocle3 | Saunders et al., 2019 PMID:31140974 | V3 | https://github.com/cole-trapnell-lab/monocle3; Trapnell et al., 2022 |
| Software, algorithm | SCANPY | La Manno et al., 2018 PMID:30089906 | V0.2.0 | https://github.com/theislab/scvelo; Theis Lab, 2017 |
| Software, algorithm | scvelo | Bergen et al., 2020 PMID:32747759 | V1.6.0 | https://github.com/theislab/scanpy; scverse, 2022 |
| Software, algorithm | NicheNetR | Browaeys et al., 2020 PMID:31819264 | https://github.com/saeyslab/nichenetr; DaMBi, 2023 | |
| Software, algorithm | cellphonedb | Garcia-Alonso et al., 2021 PMID:34857954 | https://github.com/Teichlab/cellphonedb; Teichmann Group, 2021 | |
| Other | GEO data: scRNAseq of human skin | Joost et al., 2020 PMID:23109378 | GSE129218 | |
| Other | GEO data: scRNAseq of zebrafish skin | Saunders et al., 2019 PMID:31140974 | GSE131136 | |
| Other | GEO data: scRNAseq of murine skin | Infarinato et al., 2020 PMID:33184221 | GSE147299 |
Additional files
-
Supplementary file 1
Table of single-cell RNA-sequencing cluster markers and sample statistics.
Cluster labels (Table 1, Tab 1) and top 100 differentially expressed marker genes (Table 1, Tab 2) from Figure 1C, and number of cells sampled from each condition (Table 1, Tab 3) from Figure 1A, B.
- https://cdn.elifesciences.org/articles/78942/elife-78942-supp1-v2.xlsx
-
Supplementary file 2
Table of cluster-specific differentially expressed genes.
Top 100 differentially expressed marker genes from melanocyte progenitor-0 (MP-0), melanocyte progenitor-1 (MP-1), cycling differentiation, direct differentiation, and melanocyte subclusters found in Figure 2A which were used to calculate enrichment scores on murine data in Figure 3—figure supplement 1.
- https://cdn.elifesciences.org/articles/78942/elife-78942-supp2-v2.xlsx
-
Supplementary file 3
Table of RT-PCR primer sequences.
Previously validated primer sequences for kitlga and kita utilized in Figure 3 (Hultman et al., 2007; Parichy et al., 1999).
- https://cdn.elifesciences.org/articles/78942/elife-78942-supp3-v2.xlsx
-
MDAR checklist
- https://cdn.elifesciences.org/articles/78942/elife-78942-mdarchecklist1-v2.pdf