DNALI1 interacts with the MEIG1/PACRG complex within the manchette and is required for proper sperm flagellum assembly in mice
Figures
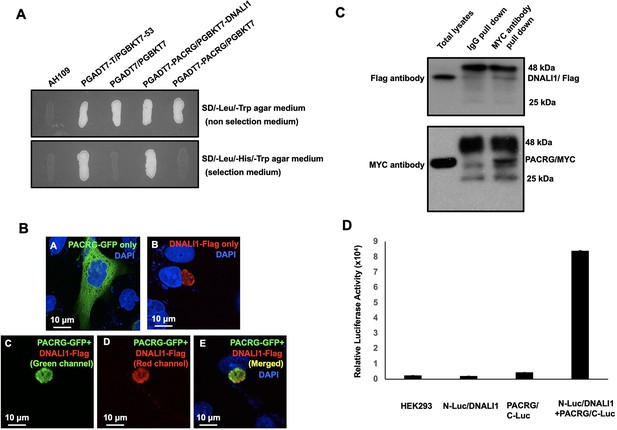
DNALI1 associates with PACRG, a major spermatogenesis regulator.
(A) Direct yeast two-hybrid assay to examine the interaction between PACRG and DNALI1. Pairs of indicated plasmids were co-transformed into AH109 yeast, and the transformed yeast were grown on either selection plates (lacking leucine, histidine, and tryptophan) or non-selection plates (lacking leucine and tryptophan). Notice that all the yeast except AH109 grew on the non-selection plate. Yeast expressing PACRG/DNALI1 and P53/large T antigen pairs grew on selection plate. (B) DNALI1 co-localizes with PACRG in Chinese hamster ovarian (CHO) cells. When expressed alone, PACRG/GFP was present in the cytoplasm, and DNALI1/FLAG as a granule located closed to the nucleus. When the two proteins were co-expressed, DNALI1/FLAG recruited the PACRG/GFP to the granule structure. Images were taken with a laser scanning confocal microscopy (Zeiss LSM 700, Virginia Commonwealth University). (C) Co-immunoprecipitation of DNALI1/FLAG with PACRG/Myc. COS-1 cells were transfected with plasmids to co-express DNALI1/FLAG and PACRG /Myc. The cell lysate was immunoprecipitated with anti-MYC antibody and then analyzed by western blotting with anti-MYC and anti-FLAG antibodies. The cell lysate immunoprecipitated with a mouse normal IgG was used as a control. The anti-MYC antibody pulled down both PACRG/MYC and DNALI1/FLAG. (D) Interaction of PACRG with DNALI1 in HEK293 cells as determined by Gaussia princeps luciferase complementation assay. HEK293 cells were transfected with the indicated plasmids, and luciferase activity was evaluated 24 hr after transfection. The cells expressing both N-Luc/DNALI1 and PACRG/C-Luc reconstituted activity.
-
Figure 1—source data 1
Co-immunoprecipitation of DNALI1/FLAG with PACRG/Myc.
- https://cdn.elifesciences.org/articles/79620/elife-79620-fig1-data1-v2.zip
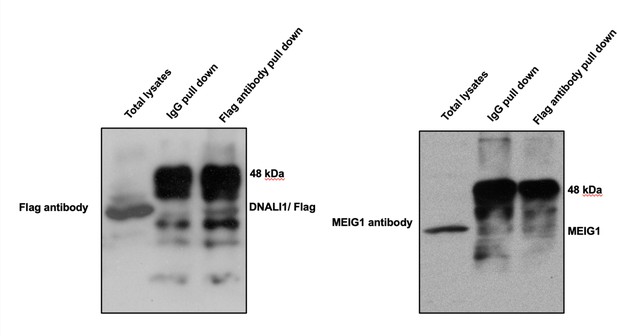
DNALI1 does not bind to MEIG1.
Co-immunoprecipitation of DNALI1/FLAG with MEIG1. COS-1 cells were transfected with plasmids to co-express DNALI1/FLAG and MEIG1. The cell lysate was immunoprecipitated with anti-Flag antibody and then analyzed by western blotting with anti-Flag and anti-MEIG1 antibodies. The cell lysate immunoprecipitated with a mouse normal IgG was used as a control. The anti-Flag antibody only pulled down DNALI1/FLAG but not MEIG1, indicating that DNALI1 does not bind to MEIG1.
-
Figure 1—figure supplement 1—source data 1
Co-immunoprecipitation of DNALI1/FLAG with MEIG1.
- https://cdn.elifesciences.org/articles/79620/elife-79620-fig1-figsupp1-data1-v2.zip
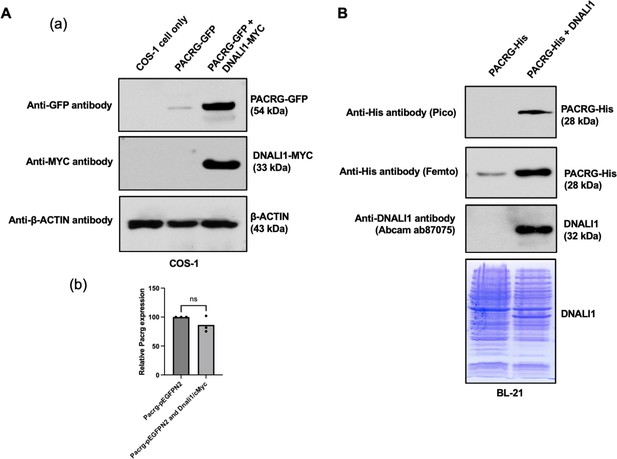
DNALI11 stabilizes PACRG in mammalian cells and bacteria.
(A) DNALI1 stabilizes PACRG in COS-1 cells. (a) Mouse PACRG-GFP expression is increased when DNALI1 is co-expressed in transfected COS-1 cells in transient expression experiment. (b) Pacrg mRNA is similar in COS-1 cells transfected with PACRG-GFP alone and PACRG-GFP and DNALI1-MYC. (B) DNALI1 stabilizes PACRG in bacteria. Notice that PACRG was only detectable by the high sensitivity Femto system in western blot analysis when the bacteria were transformed with PACRG/pCDFDuet-1 plasmid. However, when the bacteria were transformed with PACRG/DNALI1/pCDFDuet-1 plasmid to express DNALI1 protein, PACRG was also detectable by less sensitive Pico system. Total protein lysate is shown by Coomassie stain of SDS-page gel.
-
Figure 2—source data 1
DNALI11 stabilizes PACRG in mammalian cells and bacteria.
- https://cdn.elifesciences.org/articles/79620/elife-79620-fig2-data1-v2.zip
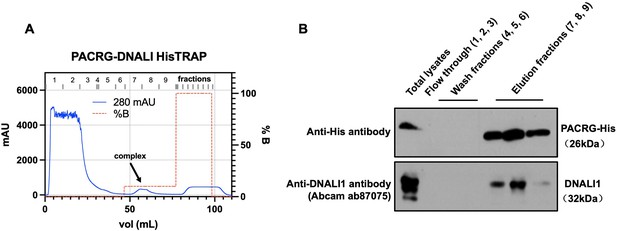
Co-purification of DNALI1 with His-tagged PACRG from bacteria lysates expressing the two proteins.
(A) His-tagged PACRG and DNALI1 were co-expressed in BL21 bacteria and His-tagged PACRG was purified from the bacteria lysates by nickel affinity chromatography. The protein complex eluted in the low imidazole wash (arrow). (B) The presence of the His-tagged PACRG and non-tagged DNALI1 in the fractions were examined by western blot analysis. Notice that the DNALI1 protein was present in the same fractions of His-tagged PACRG, indicating that the two proteins associate in the bacteria lysates.
-
Figure 3—source data 1
Co-purification of DNALI1 with His-tagged PACRG from bacteria lysates expressing the two proteins.
- https://cdn.elifesciences.org/articles/79620/elife-79620-fig3-data1-v2.zip
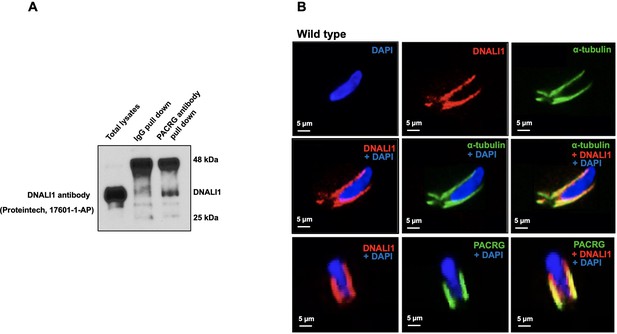
Localization of DNALI1 in male germ cells of wild-type mice.
(A) Co-immunoprecipitation assay. Mouse testis extract was pulled down using an anti-PACRG antibody, and western blot was conducted using an anti-DNALI1 antibody. Notice that DNALI1 was co-pulled down by the anti-PACRG antibody. (B) Localization of DNALI1 in isolated germ cells was examined by immunofluorescence staining. DNALI1 protein localizes with α-tubulin, a manchette marker in elongating spermatids (top and middle panels). PACRG also co-localized with DNALI1 in the elongating spermatids (bottom panel). DNALI1 seems to be closer to the nuclear membrane, and PACRG is on the surface of DNALI1. Images were taken with a laser scanning confocal microscopy (Zeiss LSM 700), Virginia Commonwealth University.
-
Figure 4—source data 1
Co-immunoprecipitation assay shows DNALI1 was co-pulled down by the anti-PACRG antibody.
- https://cdn.elifesciences.org/articles/79620/elife-79620-fig4-data1-v2.zip
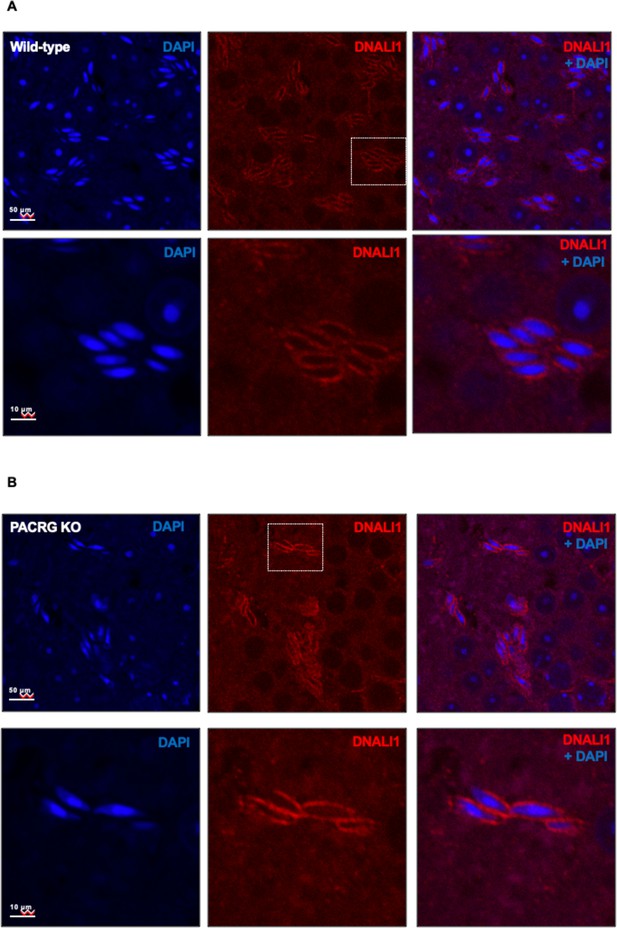
The localization of DNALI1 in the testis seminiferous tubule of a wild-type mouse (A) and a Pacrg mutant mouse (B).
DNALI1 (anti-DNALI1 antibody Abcam ab87075) was present in the manchette of elongating spermatids, and the localization was not changed in the Pacrg mutant mice. The lower panels were the zoom-in areas from the upper panels. Images were taken with a confocal laser scanning microscopy (Zeiss LSM 700, Virginia Commonwealth University).
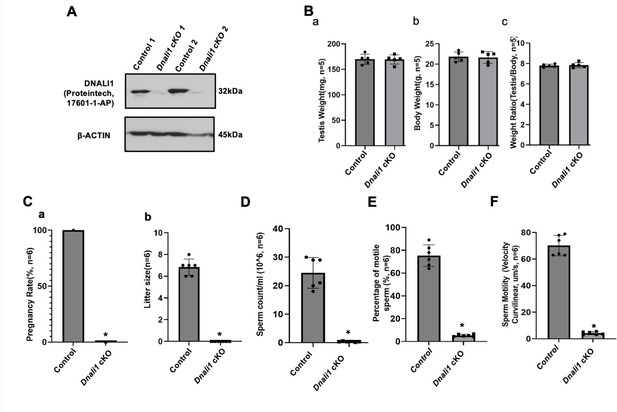
Male germ cell-specific Dnali1 knockout, Dnali1 conditional knockout (cKO) mice were infertile associated with significantly reduced sperm number and motility.
(A) Representative western blot result showing that DNALI1 protein was almost absent in the testis of Dnali1 cKO mice. (B) Testis weight (a), body weight (b) and ratio of testis/body weight (c) of 4-month-old control and Dnali1 cKO mice. There was no significant difference between the control and the Dnali1 cKO mice. n=5. (C) Male fertility of control and Dnali1 cKO mice. Six controls and six Dnali1 cKO mice were examined. Pregnancy (a) and litter size (b) were recorded for each mating. Notice that all mutant males were infertile (n=6). (D) Sperm count was significantly reduced in Dnali1 cKO mice (n=6). (E) Percentage of motile sperm in the control and Dnali1 cKO mice (n=6). (F) Sperm motility was significantly reduced in Dnali1 cKO mice (n=6). Statistically significant differences: *p < 0.05.
-
Figure 5—source data 1
Western blot result showing that DNALI1 protein was almost absent in the testis of Dnali1 conditional knockout (cKO) mice.
- https://cdn.elifesciences.org/articles/79620/elife-79620-fig5-data1-v2.zip
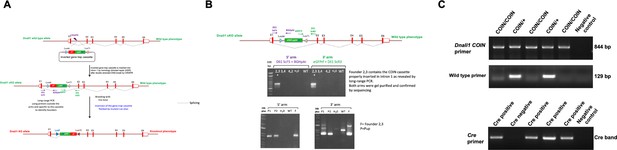
Generation of the conditional Dnali1 knockout (KO) mice.
(A) Strategy for the generation of conditional Dnali1 KO mice. (B) PCR screening to identify Dnali1COIN/+ founders and F1 pups. (C) Representative PCR results showing mice with different genotypes. Upper panel: primer set to analyze COIN Dnali1 allele (844 bp); middle panel: primer set to analyze the wild-type allele (129 bp); lower panel: primer set to detect Cre.
-
Figure 5—figure supplement 1—source data 1
Representative PCR results showing mice with different genotypes.
Upper panel: primer set to analyze COIN Dnali1 allele (844 bp); middle panel: primer set to analyze the wild-type allele (129 bp); lower panel: primer set to detect Cre.
- https://cdn.elifesciences.org/articles/79620/elife-79620-fig5-figsupp1-data1-v2.zip
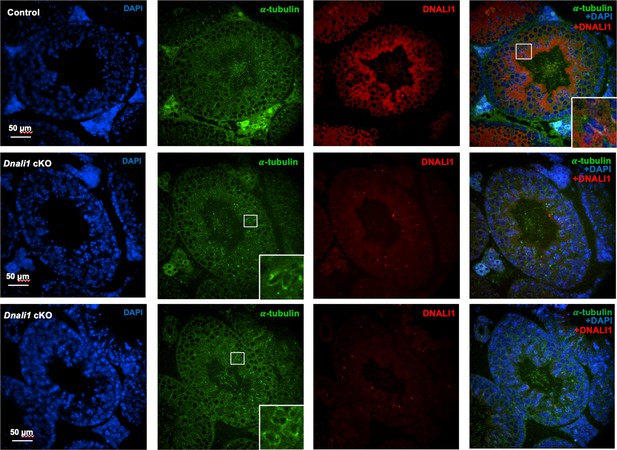
Examination of DNALI1 in testicular sections of the control and Dnali1 conditional knockout (cKO) mice.
DNALI1 (anti-DNALI1 antibody: Proteintech, 17601-1-AP) was present in the seminiferous tubules in the control mouse (upper panel), but not in the Dnali1 cKO mouse (lower two panels). Images were taken with an Olympus IX-81 microscope equipped with a spinning disc confocal unit in the Physiology Department, Wayne State University. The inserts indicated the zoom-in areas showing clear co-localization of DNALI1 and α-tubulin in the control mouse; or only α-tubulin staining but not the DNALI1 staining in the Dnali1 cKO mouse.
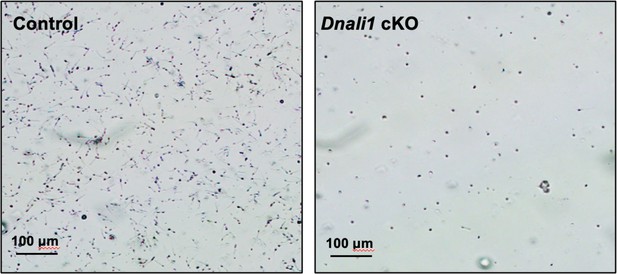
Morphological examination of epididymal sperm by light microscopy at low magnification.
Notice that sperm density of the control mice is higher than those observed in the Dnali1 conditional knockout (cKO) mice under the same dilution.
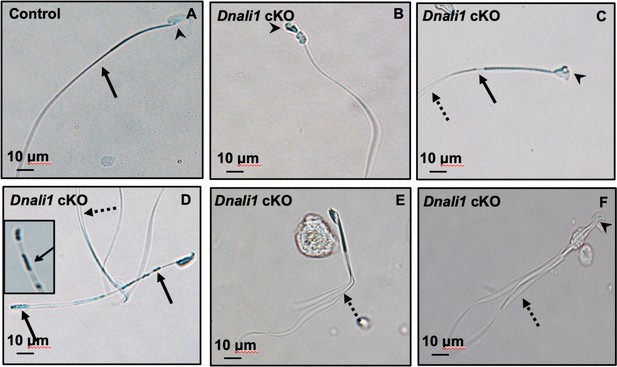
Abnormal sperm morphologies in Dnali1 conditional knockout (cKO) mice.
Representative epididymal sperm of control (A) and Dnali1 cKO mice (B–F) examined by DIC microscopy. Sperm in the control mice showed normal head (A, arrowhead) and flagella (A, arrow). Multiple abnormalities were observed in Dnali1 cKO mice, including distorted heads (B, C, and F, arrowheads), uneven thickness tails (C, D, arrows). Some sperm showed multiple flagella (C, D, E, F, dashed arrows).
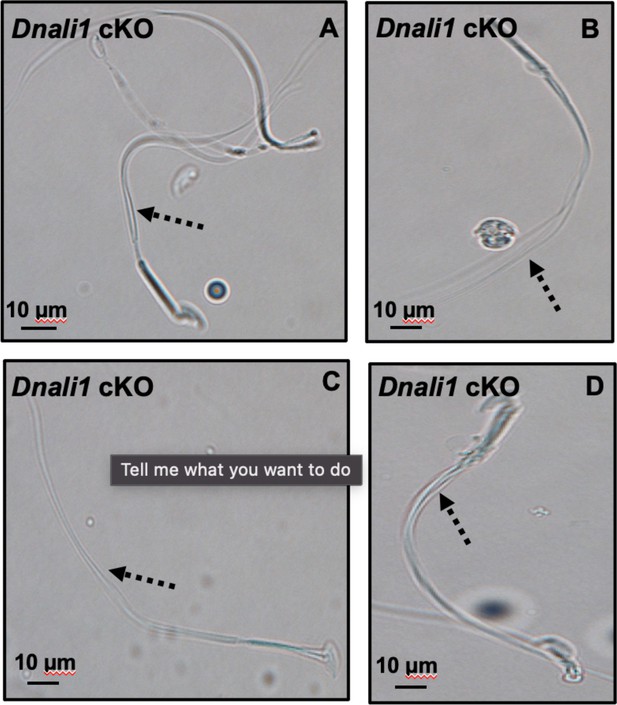
Morphological examination of epididymal sperm by light microscopy at high magnification.
Multiple tails are present in the sperm (dashed arrows in A–D).
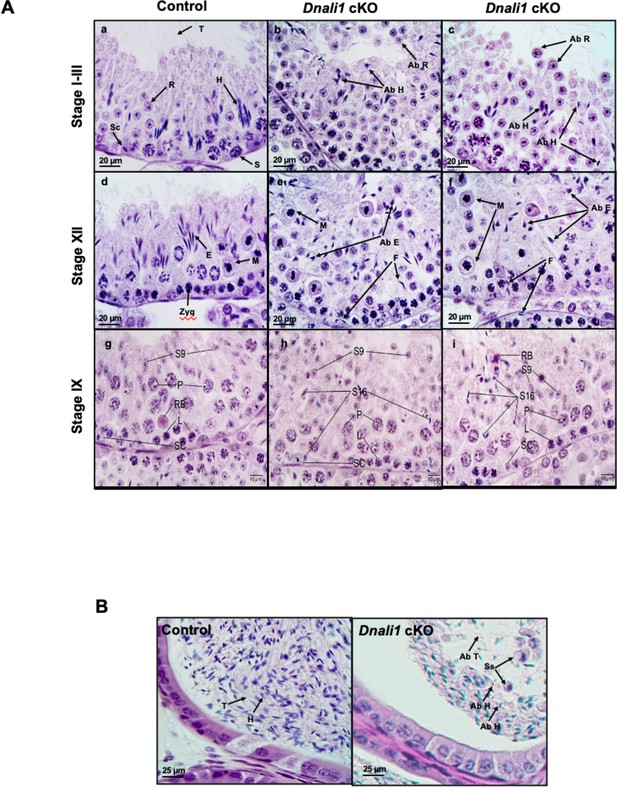
Histological analysis for the adult control and Dnali1 conditional knockout (cKO) mice.
(A) Histological evaluation of testes from control and Dnali1 cKO mice, with selected images from stages I-III (a–c), stage XII (d–f), and stage IX (g–i). (a) Control testis seminiferous tubule epithelium showing elongated spermatid heads (H) embedded as bundles among the round spermatids (R) and developing tails (T) extending into the lumen. Sc, Sertoli cell; S, spermatogonia. (b–c) Dnali1 cKO with abnormal elongating spermatid heads (Ab H) with compressed shapes and failure to form elongated bundles. Round spermatids are seen sloughing abnormally into the lumen (Ab R). (d) Control showing step 12 elongating spermatid bundles (E) with large pachytene spermatocytes in meiotic division (M). Zygotene spermatocyte, Zyg. (e–f) Dnali1 cKO with abnormal elongating spermatid heads (Ab E) without normal bundle formation. There is evidence of failure of spermiation (F), as phagocytosis of thin heads of step 16 spermatids are also present in the epithelium. Normal meiotic figures (M) are present in stage XII. (g) Control seminiferous tubule, with step 9 spermatids (S9) lining the lumen and no step 16 elongated spermatids present after spermiation. P, pachytene spermatocytes; L, leptotene spermatocytes; RB, residual body; SC, Sertoli cell. (h) Dnali1 cKO tubule showing step 9 spermatids forming (S9) along with step 16 elongated spermatids (S16) remaining at various levels within the seminiferous epithelium after failure to spermiate. P, pachytene spermatocytes; L, leptotene spermatocytes; SC, Sertoli cell. (i) A second example of a Dnali1 cKO tubule showing step 9 spermatids (S9) but numerous step 16 elongated spermatids (S16) that failed to be released into the lumen during spermiation. P, pachytene spermatocytes; L, leptotene spermatocytes; RB, residual body; SC, Sertoli cell. (B) Representative histology of epididymis. The control cauda epididymis shows the lumen filled with normal sperm heads (H) and tails (T). In Dnali1 cKO male, the cauda epididymal lumen contains numerous sperm with abnormal heads (Ab H) and tails (Ab T) and sloughed round spermatids (Ss).
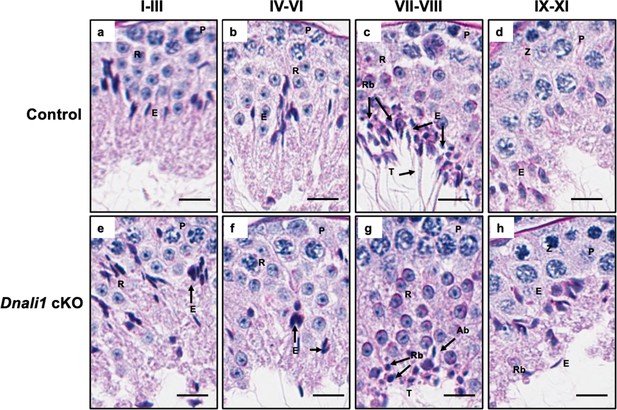
Testicular histology of adult control and Dnali1 conditional knockout (cKO) mice examined by periodic acid–Schiff (PAS) staining.
Testes were collected from 3- to 4-month-old control and Dnali1 cKO mice and fixed in 4% paraformaldehyde (PFA). The seminiferous tubules were stained by PAS. (a–d) Spermatogenesis is normal in the control mice. P, pachytene spermatocytes; RB, residual body; E, elongating spermatid; R, round spermatid; SC, Sertoli cell. Bar = 20 μm for all images. (e–h) Abnormal spermatogenesis stages in Dnali1 cKO mice. P, pachytene spermatocytes; RB, residual body; E, elongating spermatid; R, round spermatid; Ab, abnormal spermatid; SC, Sertoli cell. Bar = 20μm for all images.
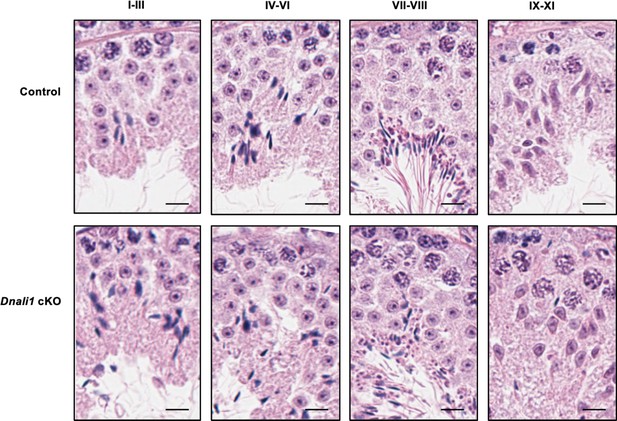
Testicular histology of adult control and Dnali1 conditional knockout (cKO) mice examined by hematoxylin and eosin (H&E) staining.
The testes were fixed in Bouin’s solution and H&E staining was conducted. Bar = 20μm for all images.
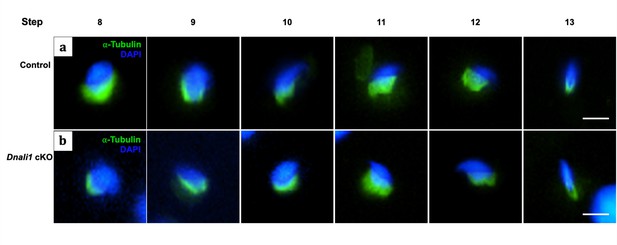
The manchette architecture is similar between the control and Dnali1 conditional knockout (cKO) mice in isolated germ cells.
The testicular cells were isolated from the control and Dnali1 cKO mice, and immunofluorescence staining was conducted using an anti-α-tubulin (green) antibody. The nuclei were stained with DAPI (blue). Notice that the distribution of α-tubulin in elongating spermatids was similar between control (a) and Dnali1 cKO (b) mice. Scale bar: 5 µm. Images were taken under a Nikon DS-Fi2 Eclipse 90i Motorized Upright Fluorescence Microscope (The C.S. Mott Center for Human Growth and Development, Department of Obstetrics & Gynecology, Wayne State University).
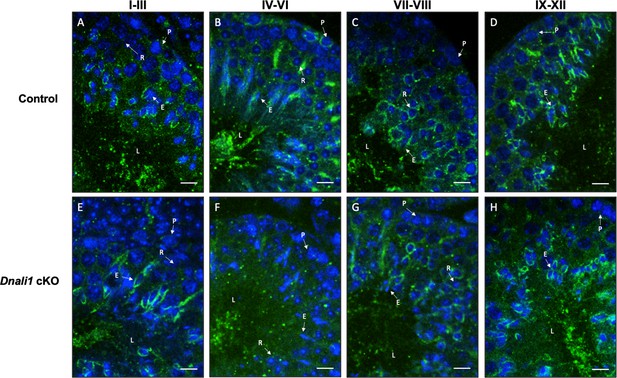
Examination of manchette structure in the testis seminiferous tubules of the control and Dnali1 conditional knockout (cKO) mice.
Testis immunofluorescence from control (A–D) and Dnali1 cKO mice (E–H). P, pachytene spermatocytes; E, elongating spermatid; R, round spermatid; L, lumen. Bar = 20 μm for all images. Images were taken with an Olympus IX-81 microscope equipped with a spinning disc confocal unit in the Physiology Department, Wayne State University.
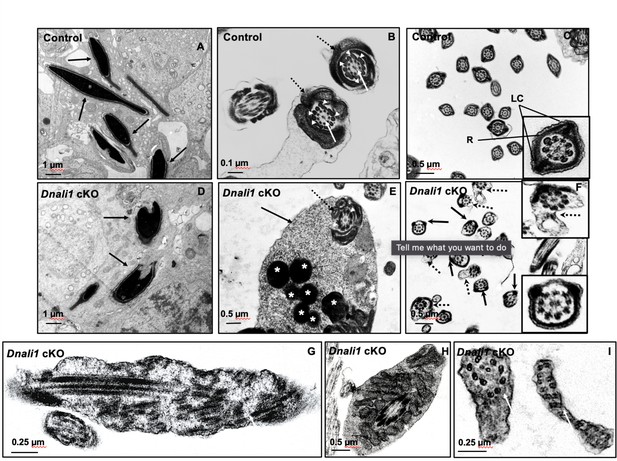
Ultrastructural changes of testicular sperm of control and Dnali1 conditional knockout (cKO) mice.
The ultrastructure of testicular sperm from the control (A, B, and C) and Dnali1 cKO (D–I) mice were analyzed by transmission electron microscopy (TEM). (A) Control testis seminiferous tubule epithelium showing nuclei with normally condensed chromatin (arrows). (B) Control mouse showed the normal midpiece of flagella with normal ‘9+2’ axoneme structure in the center (white arrows) surrounded by mitochondrial sheath (black dotted arrows) and ODF (white arrow heads). (C) Normal principal piece of flagella from a control mouse, which is characterized by the presence of a complete fibrous sheath surrounding the axoneme. The fibrous sheath consists of two longitudinal columns (LC) connected by semicircumferential ribs (R). The two longitudinal columns are associated with microtubule doublets 3 and 8, and the two semicircumferential ribs are symmetrical. (D) Abnormally condensed chromatin in Dnali1 cKO mouse (black arrows). (E) A representative image of the midpiece in a flagellum from a Dnali1 cKO mouse. The ODF (white arrow heads) and mitochondrial sheath (black dotted arrow) were present, but cytoplasm residue (black arrow) remained with a number of the lysosomes inside (white stars). (F) The flagella show disorganized fibrous sheath structure in the Dnali1 cKO mouse. Noticed that the two longitudinal columns are not associated with microtubule doublets 3 and 8 in some flagella, and the two semicircumferential ribs showing defective or asymmetric organization (black arrows and the lower, right insert); some flagella also have disrupted membranes (dashed arrows and upper, right insert). (G–I) The flagella show disrupted axonemes in Dnali1 cKO mice (white arrows).
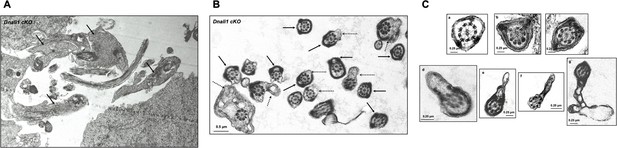
Additional testicular sperm transmission electron microscopy (TEM) images of the Dnali1 conditional knockout (cKO) mice.
(A) Low magnification image showing retained cytoplasmic components in the lumen area (black arrows). (B) Low magnification images showing abnormal fibrous sheath (black arrows) and cell membranes (dashed arrows). (C) High magnification images showing abnormal fibrous sheath (a–c) and cell membranes (d–g).
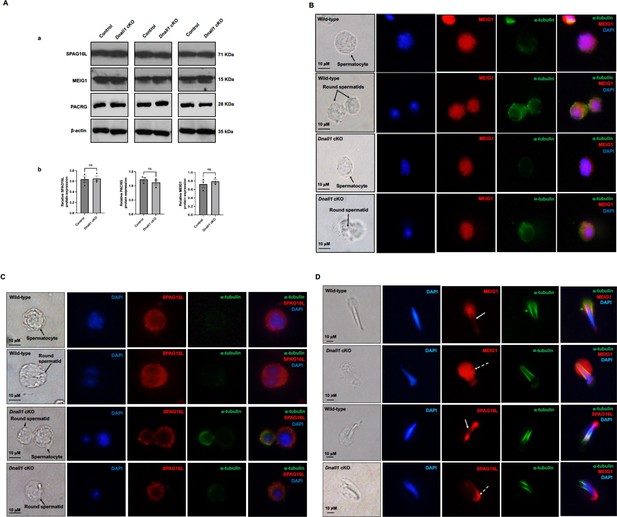
Expression levels and localization of DNALI1 downstream proteins in the Dnali1 conditional knockout (cKO) mice.
(A) Analysis of testicular expression of MEIG1, PACRG, and SPAG16L in control and Dnali1 cKO mice by western blot. Compared with control mice, there was no significant change in the expression level of these three proteins in Dnali1 cKO mice. (a) Representative western blot results. (b) Statistical analysis of the protein levels normalized by β-actin. n=4. (B) Localization of MEIG1 in spermatocytes and round spermatids of the control and Dnali1 cKO mice by immunofluorescence staining. There is no difference between the control and Dnali1 cKO mice. MEIG1 is present in cell bodies in both genotypes. (C) Localization of SPAG16L in spermatocytes and round spermatids of the control and Dnali1 cKO mice by immunofluorescence staining. There is no difference between the control and the Dnali1 cKO. SPAG16L is present in cell bodies in both genotypes. The white dashed arrow points to an elongating spermatid, and the SPAG16L is present in the manchette. (D) Localization of MEIG1 and SPAG16L in elongating spermatids of the control and Dnali1 cKO mice. Both MEIG1 and SPAG16L are present in the manchette in the control mice (white arrows); however, they are no longer present in the manchette in Dnali1 cKO mice (dashed white arrows). Images were taken under a Nikon DS-Fi2 Eclipse 90i Motorized Upright Fluorescence Microscope (The C.S. Mott Center for Human Growth and Development, Department of Obstetrics & Gynecology, Wayne State University).
-
Figure 10—source data 1
Expression levels of DNALI1 downstream proteins in the Dnali1 conditional knockout (cKO) mice.
- https://cdn.elifesciences.org/articles/79620/elife-79620-fig10-data1-v2.zip
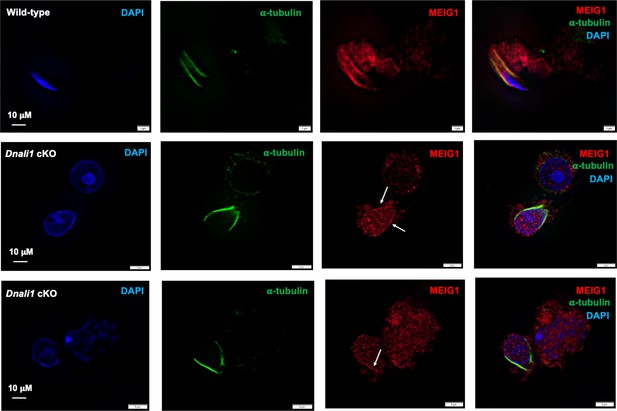
Examination of MEIG1 localization in elongating spermatids in the control and Dnali1 conditional knockout (cKO) mice.
Notice that MEIG1 is present in the manchette in the control mouse. In the Dnali1 cKO mouse, a trace amount of MEIG1 appears to be present in the manchette. Images were taken with Olympus IX-81 microscope equipped with a spinning disc confocal unit in Dr. James G. Granneman’s laboratory, Wayne State University. The white arrows point to the trace amount of MEIG1 in the manchette.
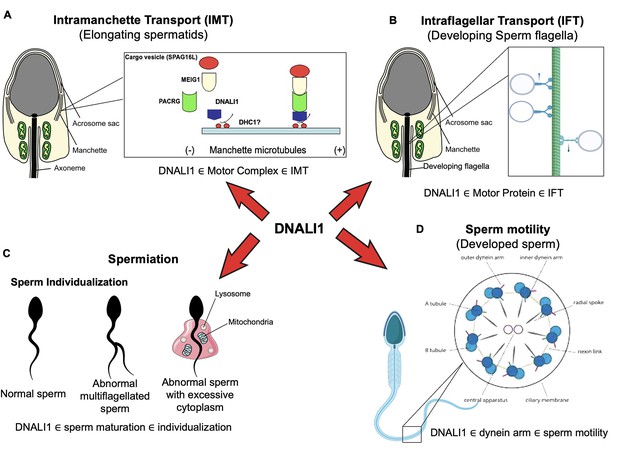
Working model of DNALI1 in sperm cell differentiation and function.
(A) DNALI1 forms a complex with MEIG1/PACRG, with DNALI1 being an upstream protein that recruits downstream PACRG and MEIG1 to the manchette. DNALI1 associates with the manchette microtubules through other molecular motor protein(s), including dynein heavy chain 1. MEIG1/PACRG/DNALI1/motor complex transports cargos, including SPAG16L, along the manchette to build sperm flagella. (B) DNALI1 might also function as a motor protein involved in transporting intraflagellar transport (IFT) particles. (C) DNALI1 may facilitate in the appropriate maturation and individualization of sperm cells. (D) DNALI1 is present in the dynein arm and functions in sperm motility.
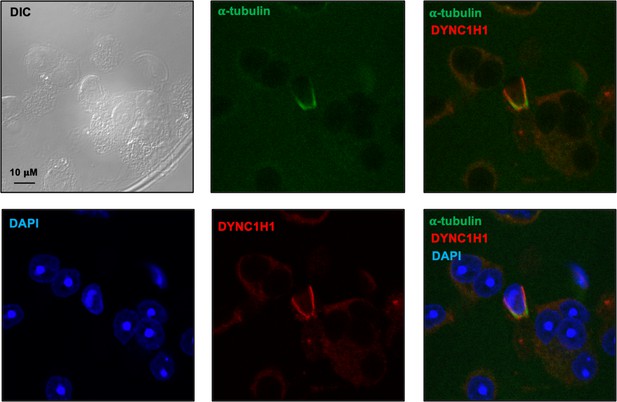
Examination of dynein heavy chain 1 protein (DYNC1H1) in male germ cells by immunofluorescence staining.
Notice that DYNC1H1 was co-localized with α-tubulin in the elongating spermatid. Images were taken with a confocal laser scanning microscopy (Zeiss LSM 700, Virginia Commonwealth University).
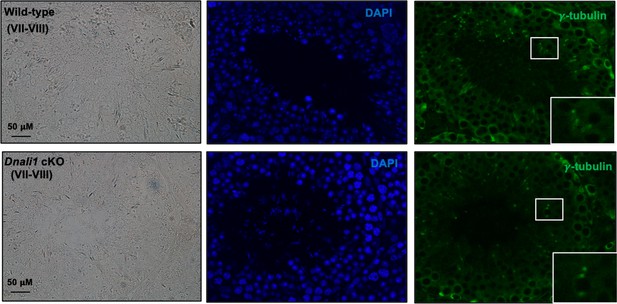
Basal body/centriole formation was not affected in the absence of DNALI1 in male germ cells.
IF was conducted on the testicular sections from the control and Dnali1 conditional knockout (cKO) mice using an anti-γ-tubulin antibody. Notice that there was no difference in γ-tubulin localization pattern, indicating that the basal body/centriole formation was not affected.
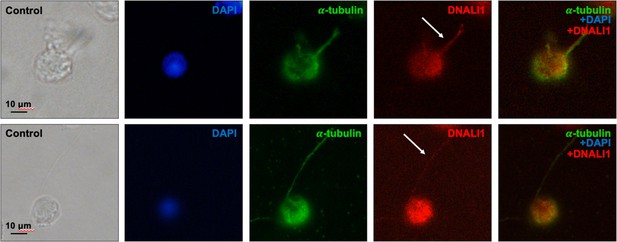
DNALI1 is present in the developing mouse sperm flagella.
Testicular cells were isolated from a wild-type mouse, and immunofluorescence staining was conducted using an anti-DNALI1 antibody (Proteintech, 17601-1-AP) and an anti-α-tubulin antibody. DNALI1 was present in the developing sperm flagella (white arrows).
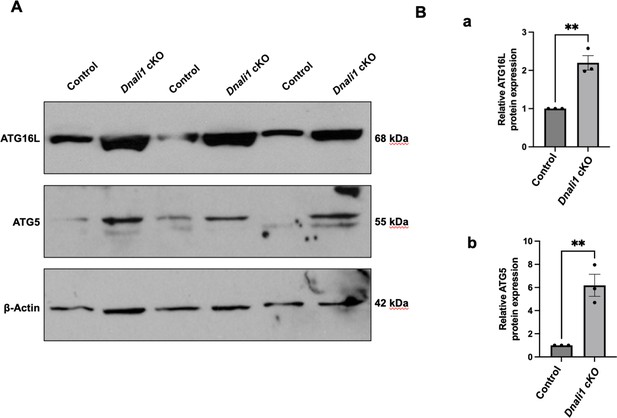
Inactivation of Dnali1 in male germ cells resulted in abnormal autophagy pathway.
Western blot analysis of selective core autophagy components, including ATG16L and ATG5 in testes of the control and Dnali1 conditional knockout (cKO) mice. Compared with control mice, there was a significant increase in the expression level of these two proteins in the Dnali1 cKO mice. (A) Representative western blot results. (B) Statistical analysis of the protein levels (a: ATG16L; b: ATG5) normalized by β-actin. n=3.
-
Figure 11—figure supplement 4—source data 1
Western blot analysis of selective core autophagy components, ATG16L and ATG5.
- https://cdn.elifesciences.org/articles/79620/elife-79620-fig11-figsupp4-data1-v2.zip
Videos
Representative movie from a control mouse.
Note that most sperm are motile and display vigorous flagellar activity and progressive long-track forward movement.
Representative movies from a Dnali1 cKO mouse.
Notice that there are fewer sperm compared with the control mice in the same dilution, and almost all sperm are immotile.
Tables
List of putative PACRG binding proteins selected under stringent conditions.
The full-length PACRG coding sequence was cloned into pGBKT7, which was used to screen a Mate & Plate Library-Universal Mouse (Normalized) (Clontech, Cat No: 630482) according to the manufacturer’s instructions. The yeasts were grown on plates lacking four amino acids (Ade-Leu-His-Trp). DNALI1 was found to be one of the putative PACRG binding proteins.
| Name | NCBI number | Frequency |
|---|---|---|
| Meig1 | NM_008579 | 119 |
| Dnali1 | NM_175223 | 6 |
| Pramel42 | NM_001243938 | 3 |
| Musculus protein phosphatase 1A | BC008595 | 2 |
| Acad11 | NM_175324 | 2 |
| Ppm1a | NM_008910 | 1 |
| L2hgdh | NM_145443 | 1 |
| Tmem225 | NM_029379 | 1 |
| Tinag | NM_012033 | 1 |
| Spag6l | NM_015773 | 1 |
| Emp2 | NM_007929 | 1 |





