Regulation of store-operated Ca2+ entry by IP3 receptors independent of their ability to release Ca2+
Figures
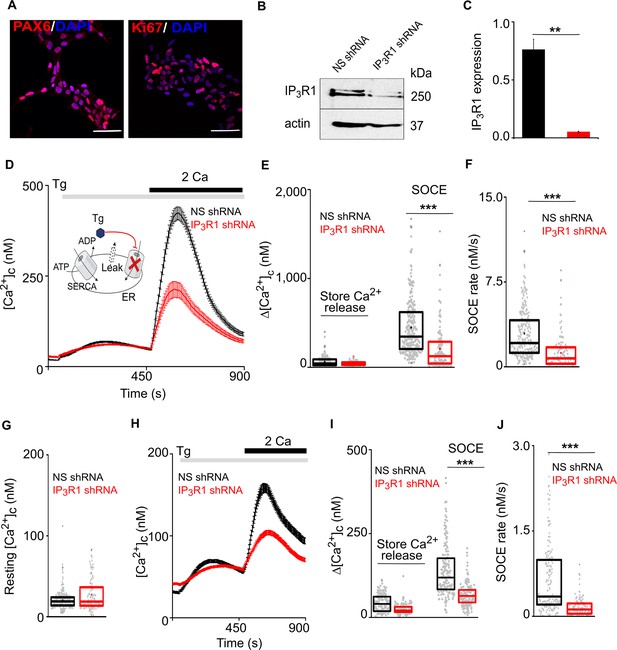
Loss of IP3R1 attenuates SOCE in human neural stem cells.
(A) Confocal images of hNPCs (passage 6) stained for DAPI and neural stem cell proteins: Pax6 and Ki67 (proliferation marker). Scale bars, 50 μm. (B) WB for IP3R1 of hNPCs expressing non-silencing (NS) or IP3R1-shRNA. (C) Summary results (mean ±s.d., n=3) show IP3R1 expression relative to actin. **p < 0.01, Student’s t-test with unequal variances. (D) Changes in [Ca2+]c evoked by thapsigargin (Tg, 10 µM) in Ca2+-free HBSS and then restoration of extracellular Ca2+ (2 mM) in hNPCs expressing NS or IP3R1-shRNA. Mean ± s.e.m. from hree independent experiments, each with four replicates that together included 100–254 cells. Inset shows the target of Tg. (E–G) Summary results (individual cells, median (bar), 25th and 75th percentiles (box) and mean (circle)) show Ca2+ signals evoked by Tg or Ca2+ restoration (E), rate of Ca2+ entry (F) and resting [Ca2+]c (G). ***p < 0.001, Mann-Whitney U-test. (H) Changes in [Ca2+]c evoked by Tg (10 µM) in Ca2+-free HBSS and after restoring extracellular Ca2+ (2 mM) in neurons (differentiated hNPCs) expressing NS or IP3R1-shRNA. Mean ± s.e.m. from three experiments with ~200 cells. (I,J) Summary results (presented as in E-G) show Ca2+ signals evoked by Tg or Ca2+ restoration (I) and rate of Ca2+ entry (J). ***p < 0.001. Mann-Whitney U-test. See also Figure 1—figure supplement 1. Source data in Figure 1—source data 1.
-
Figure 1—source data 1
Loss of IP3R1 attenuates SOCE in human neural stem cells.
- https://cdn.elifesciences.org/articles/80447/elife-80447-fig1-data1-v2.zip
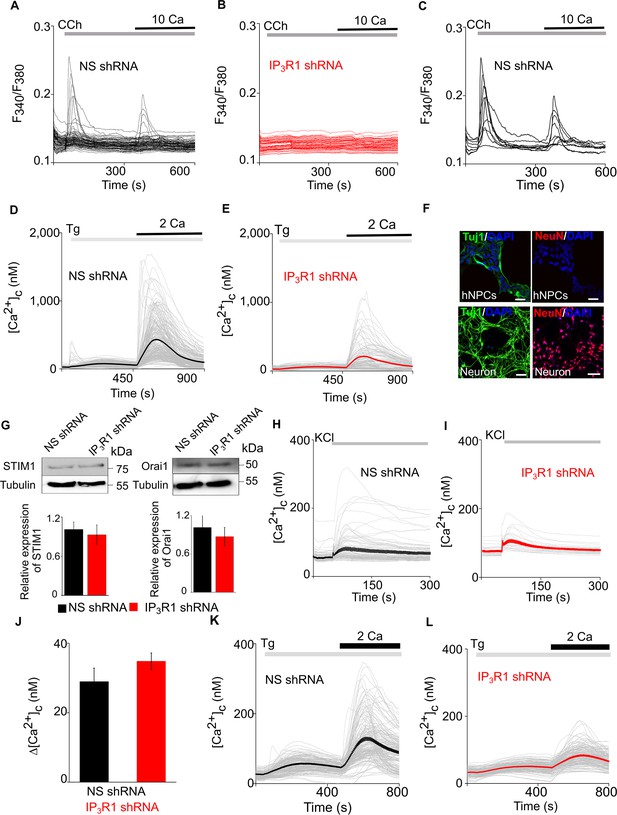
Loss of IP3R1 attenuates SOCE in neural precursor cells and differentiated neurons.
(A–C) hNPCs expressing NS (A, C) or IP3R1-shRNA (B) were stimulated with carbachol (CCh, 100 µM) in Ca2+-free HBSS before restoring extracellular Ca2+ (10 mM). Each trace shows the Fura 2 fluorescence ratio (F340/F380) from a single cell (>50 cells from three experiments). Traces in (C) show only cells that responded to CCh. (D, E) hNPCs expressing shRNA were stimulated with thapsigargin (Tg, 10 µM) in Ca2+-free HBSS before restoring extracellular Ca2+ (2 mM). Traces are from individual cells (>180 cells from three experiments), with the mean response shown by a thick line. Summary results in Figure 1D–G. (F) Confocal images of hNPCs and neurons spontaneously differentiated (10 days) from hNPCs stained for DAPI and neuronal markers: Tuj1 (βIII-tubulin) and NeuN (neuronal nuclear antigen). Scale bars, 50 μm. Only differentiated hNPCs express neuronal markers. (G) WB for STIM1 and Orai1 in lysates from hNPCs cells expressing non-silencing (NS) or IP3R1-shRNA. Summary results (mean ±s.d., n=3) show relative expression of STIM1 and Orai1 relative to control shRNA (NS) cells. p>0.01, Student’s t-test with unequal variances. (H, I) Spontaneously differentiated hNPCs (25 days) expressing NS (H) or IP3R1-shRNA (I) were depolarized by addition of KCl (75 mM). Traces show responses from single cells (>30 cells from three experiments) and mean response (thick line). (J) Summary results (mean ± s.e.m., three experiments) show peak increases in [Ca2+]c (Δ[Ca2+]c) evoked by depolarization. No significant difference, Student’s t-test with unequal variances. (K, L) Spontaneously differentiated hNPCs expressing NS (K) or IP3R1-shRNA (L) were stimulated with Tg (10 µM) in Ca2+-free HBSS before restoring extracellular Ca2+ (2 mM). Traces from individual cells (>100 cells from three experiments) and the mean response (bold) are shown. Summary results in Figure 1H–J. Source data in Figure 1—figure supplement 1—source data 1.
-
Figure 1—figure supplement 1—source data 1
Loss of IP3R1 attenuates SOCE in neural precursor cells and differentiated neurons.
- https://cdn.elifesciences.org/articles/80447/elife-80447-fig1-figsupp1-data1-v2.zip
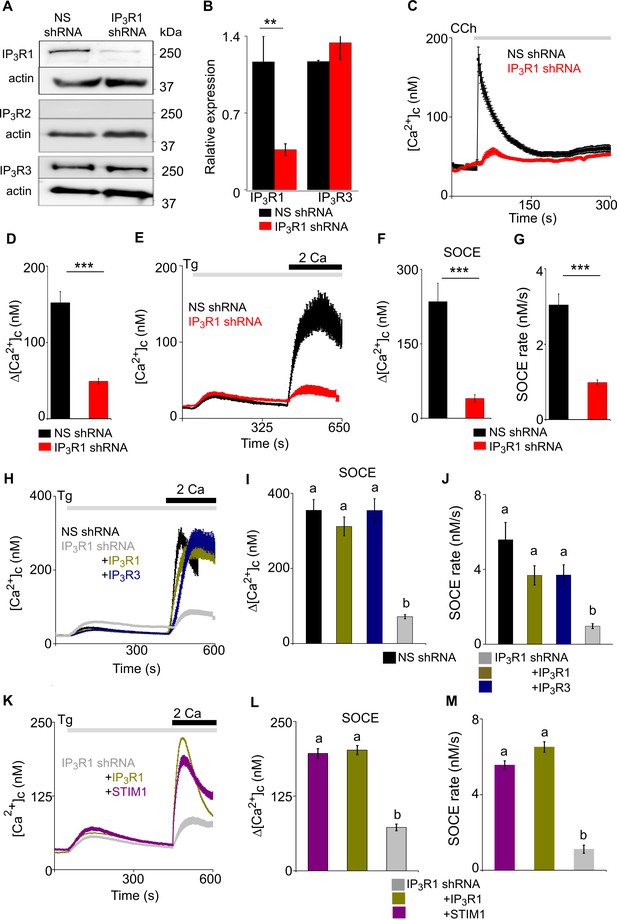
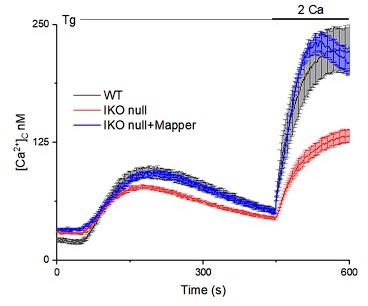
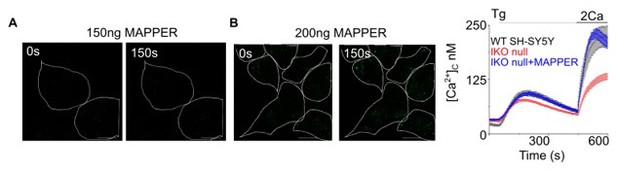
Loss of IP3R1 attenuates SOCE in SH-SY5Y cells.
(A) WB for IP3R1-3 of SH-SY5Y cells expressing non-silencing (NS) or IP3R1-shRNA. (B) Summary results (mean ± s.d., n=4) show IP3R expression relative to actin normalized to control NS cells. **p < 0.01, Student’s t-test with unequal variances. (C) Ca2+ signals evoked by carbachol (CCh, 3 µM) in SH-SY5Y cells expressing NS or IP3R1-shRNA. Mean ± s.e.m. from three experiments with 70–90 cells. (D) Summary results show peak changes in [Ca2+]c (Δ[Ca2+]c) evoked by CCh. ***p < 0.001, Mann-Whitney U-test. (E) Ca2+ signals evoked by thapsigargin (Tg, 10 µM) in Ca2+-free HBSS and then after restoration of extracellular Ca2+ (2 mM) in cells expressing NS or IP3R1-shRNA. Mean ± s.e.m. from three experiments with ~50 cells. (F, G) Summary results (individual cells, mean ± s.e.m., n=3, ~50 cells) show peak changes in [Ca2+]c evoked by Ca2+ restoration (Δ[Ca2+]c) (F) and rate of Ca2+ entry (G). ***p < 0.001, Mann-Whitney U-test. (H) Ca2+ signals evoked by Tg and then Ca2+ restoration in cells expressing NS-shRNA, or IP3R1-shRNA alone or with IP3R1 or IP3R3. Traces show mean ± s.e.m. (50–115 cells from three experiments). (I, J) Summary results (mean ± s.e.m, 50–115 cells from three experiments) show peak increases in [Ca2+]c (Δ[Ca2+]c) evoked by Ca2+ restoration (I) and rates of Ca2+ entry (J) evoked by restoring extracellular Ca2+. (K) Effects of thapsigargin (Tg, 10 µM) in Ca2+-free HBSS and then after Ca2+ restoration (2 mM) in cells expressing IP3R1-shRNA alone or with IP3R1 or mCh-STIM1. Traces show mean ± s.e.m. (100–150 cells from three experiments). (L, M) Summary results (mean ± s.e.m.) show peak increase in [Ca2+]c after Ca2+ restoration (Δ[Ca2+]c) (L) and rate of Ca2+ entry (M). Different letters indicate significant differences (panels I, J, L, M), p <0.001, one-way ANOVA with pair-wise Tukey’s test. See also Figure 2—figure supplements 1–3. Source data in Figure 2—source data 1.
-
Figure 2—source data 1
Loss of IP3R1 attenuates SOCE in SH-SY5Y cells.
- https://cdn.elifesciences.org/articles/80447/elife-80447-fig2-data1-v2.zip
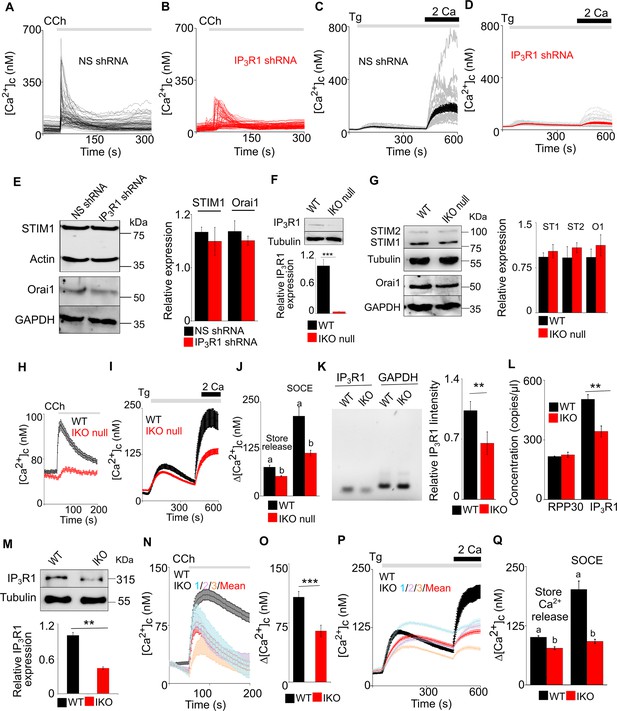
Reduced expression of IP3R1 using either shRNA or CRISPR/Cas9n attenuates SOCE in SH-SY5Y cells.
(A, B) Ca2+ signals evoked by carbachol (CCh, 3 μM) in Ca2+-free HBSS in SH-SY5Y cells expressing NS (A) or IP3R1-shRNA (B). Traces show responses from single cells (100–200 cells from three experiments). (C, D) SH-SY5Y cells expressing NS (C) or IP3R1-shRNA (D) were treated with thapsigargin (Tg, 1 µM) in Ca2+-free HBSS before restoration of extracellular Ca2+ (2 mM). Traces show responses from single cells (50–100 cells from three experiments) and mean response (thick lines). Summary results in Figure 2C–G. (E) Western blots (WB) for STIM1 and Orai1 from lysates of SH-SY5Y cells expressing non-silencing (NS) or IP3R1-shRNA. Summary results (mean ±s.d., n=3) show STIM1 and Orai1 expression relative to NS-shRNA cells. p>0.05, Student’s t-test with unequal variances. (F) Western blot for IP3R1 and tubulin from lysates from WT SH-SY5Y cells and for cells from a cell line in which the IP3R1 gene was targeted using CRISPR/Cas9 (IKO null). The blot is typical of three similar analyses. Summary results (mean ±s.d., n=3) show IP3R1 expression relative to WT cells. p<0.001, Student’s t-test with unequal variances.(G) Western blots (WB) for STIM1 (S1), STIM2 (S2) and Orai1 (O1) from lysates of WT and IKO null SH-SY5Y cells. Summary results (mean ±s.d., n=3) show STIM1,2 and Orai1 expression relative to WT cells. p>0.05, Student’s t-test with unequal variances.(H) Ca2+ signals evoked by carbachol (CCh, 1 μM) in WT and IKO null SH-SY5Y cells in Ca2+-free HBSS. (I) SH-SY5Y cells (WT or IKO null) were treated with thapsigargin (Tg, 1 µM) in Ca2+-free HBSS before restoring extracellular Ca2+ (2 mM). Mean ± s.e.m. from three experiments with WT cells (black, 110 cells), IKO null cells (red, 117 cells). (J) Summary results show the peak increases in [Ca2+]c evoked by Tg (1 µM) in Ca2+-free HBSS (Δ[Ca2+]c) and after Ca2+ restoration (SOCE). Different letters indicate p<0.001, Mann-Whitney U-test. (K) PCRs for IP3R1 and GAPDH (control gene) of genomic DNA isolated from SH-SY5Y cells. Results (mean ± s.e.m. from three independent analyses of a single cell line) are shown for wild type (WT) cells and for cells in which the IP3R1 gene was targeted using CRISPR/Cas9n (IKO). Expression of IP3R1 has been normalized to expression of GAPDH. **p < 0.01, Student’s t-test with unequal variances. (L) Droplet digital PCR amplification was used to determine gene copy number for IP3R1 and a control gene RPP30 for WT and IKO SH-SY5Y cells. **p<0.05, Student’s t-test with unequal variances. (M) Western blot for IP3R1 and tubulin from lysates from WT SH-SY5Y cells and for cells from a cell line in which the IP3R1 gene was targeted using CRISPR/Cas9n (IKO). The blot is typical of 3 similar analyses. Summary results (mean ±s.d., n=3) show IP3R1 expression relative to WT cells. **p<0.01, Student’s t-test with unequal variances. (N) Ca2+ signals evoked by carbachol (CCh, 1 μM) in WT and IKO SH-SY5Y cells in Ca2+-free HBSS. Results show (mean ± s.e.m.) for each of three independently edited cell lines (IKO1, 2 and 3), each with at least 30 cells; and the overall mean ± s.e.m. (red line). Results from a total of 89 WT cells and 130 IKO cells are shown. (O) Summary results (mean ± s.e.m. from three experiments with 80–100 cells) show peak changes in [Ca2+]c (Δ[Ca2+]c) evoked by CCh. ***p<0.001, Mann-Whitney U-test. (P) SH-SY5Y cells (WT or IKO) were treated with thapsigargin (Tg, 1 µM) in Ca2+-free HBSS before restoring extracellular Ca2+ (2 mM). Mean ± s.e.m. from three experiments with WT cells (black, 85 cells), three independently edited cell lines (IKO 1, 2 and 3; at least 30 cells for each) and the pooled results from all edited cells (97; red). (Q) Summary results show the peak increases in [Ca2+]c evoked by Tg (1 µM) in Ca2+-free HBSS (Δ[Ca2+]c) and after Ca2+ restoration (SOCE). Different letters indicate p<0.001, Mann-Whitney U-test. Source data in Figure 2—figure supplement 1—source data 1 .
-
Figure 2—figure supplement 1—source data 1
Reduced expression of IP3R1 using either shRNA or CRISPR/Cas9n attenuates SOCE in SH-SY5Y cells.
- https://cdn.elifesciences.org/articles/80447/elife-80447-fig2-figsupp1-data1-v2.zip
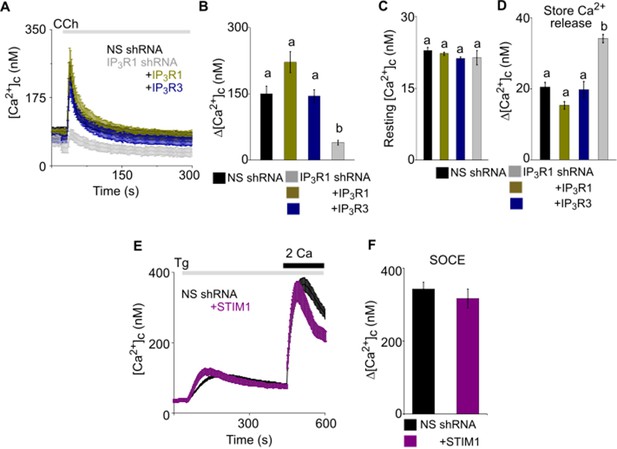
Attenuated SOCE in SH-SY5Y cells lacking IP3R1 is rescued by expression of IP3R1, IP3R3 or STIM1.
(A) Ca2+ signals evoked by carbachol (CCh, 3 μM) in SH-SY5Y cells expressing NS or IP3R1-shRNA, and the latter with IP3R1 or IP3R3. Traces show mean ± s.e.m. from >3 experiments with 50–70 cells. (B–D) Summary results show peak Ca2+ signals (Δ[Ca2+]c) evoked by CCh (B), resting [Ca2+]c (C) and the peak Ca2+ signal evoked by Tg (10 µM) in Ca2+-free HBSS (Δ[Ca2+]c, a reporter of ER Ca2+ content) (D). Different letter codes indicate significantly different values, p<0.001, one-way ANOVA and pair-wise Tukey’s test. (E) Effects of expressing mCh-STIM1 in SH-SY5Y cells expressing NS shRNA on the Ca2+ signals evoked by Tg (1 µM) in Ca2+-free HBSS and then after restoration of extracellular Ca2+ (2 mM). Traces show mean ± s.e.m. from three experiments with 30–110 cells. (F) Summary results show the peak Δ[Ca2+]c evoked by restoring extracellular Ca2+ after Tg. Mean ± s.e.m. p>0.05, Mann-Whitney U-test. Source data in Figure 2—figure supplement 2—source data 1.
-
Figure 2—figure supplement 2—source data 1
Attenuated SOCE in SH-SY5Y cells lacking IP3R1 is rescued by expression of IP3R1, IP3R3 or STIM1.
- https://cdn.elifesciences.org/articles/80447/elife-80447-fig2-figsupp2-data1-v2.zip
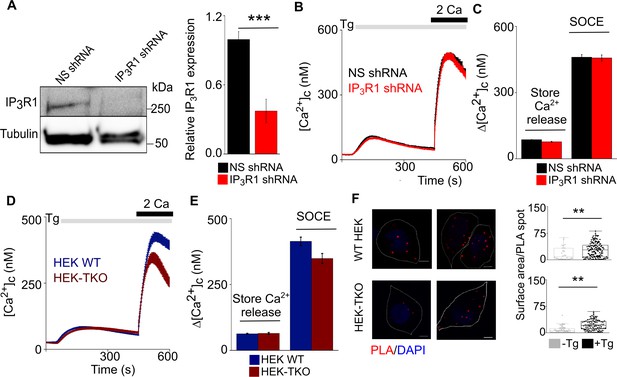
Loss of IP3R1 does not affect SOCE in HEK cells.
(A) WB for IP3R1 in HEK cells expressing non-silencing (NS) or IP3R1-shRNA. Summary results (mean ±s.d., n=3) show IP3R1 expression relative to tubulin. ***p < 0.001, Student’s t-test with unequal variances. (B) HEK cells expressing NS or IP3R1-shRNA were treated with thapsigargin (Tg, 1 µM) in Ca2+-free HBSS before restoration of extracellular Ca2+ (2 mM). Traces show mean response from >100 cells from three experiments. (C) Summary results show the peak increases in [Ca2+]c evoked by Tg (1 µM) in Ca2+-free HBSS (Δ[Ca2+]c, a reporter of ER Ca2+ content) and after Ca2+ restoration (SOCE). (D) Wild type HEK cells (HEK WT) and cells lacking all IP3Rs (HEK-TKO) were treated with thapsigargin (Tg, 1 µM) in Ca2+-free HBSS before restoration of extracellular Ca2+ (2 mM). Traces show mean ± s.e.m. from three experiments with 80–120 cells. (E) Summary results show the peak Δ[Ca2+]c evoked by restoring extracellular Ca2+ after Tg. Mean ± s.e.m. p>0.01, Mann-Whitney U-test. (F) Proximity ligation assay (PLA) analyses of interactions between STIM1 and Orai1 in wild type HEK (WT) and TKO cells. Confocal images are shown for control cells (-Tg) or after 10 min treatment with thapsigargin (+Tg, 1 µM) in Ca2+-free HBSS. PLA reaction product is red, and nuclei are stained with DAPI (blue). Scale bars, 5 µm. Summary results show the surface area of the PLA spots for ~20 cells from two independent analyses. Individual values, median (bar) and 25th and 75th percentiles (box). **p<0.01, Student’s t-test with unequal variances. Source data in Figure 2—figure supplement 3—source data 1.
-
Figure 2—figure supplement 3—source data 1
Loss of IP3R1 does not affect SOCE in HEK cells.
- https://cdn.elifesciences.org/articles/80447/elife-80447-fig2-figsupp3-data1-v2.zip
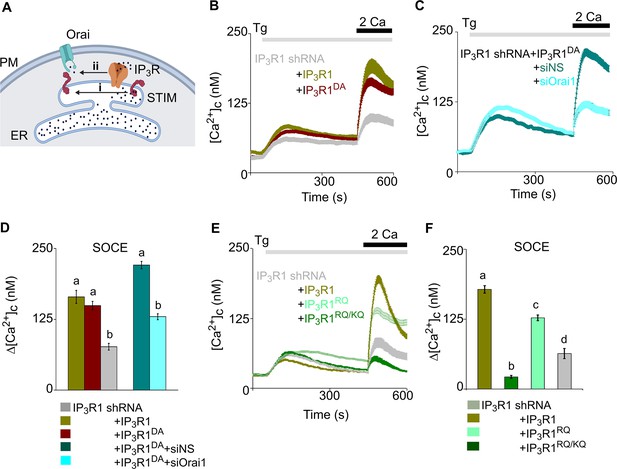
Regulation of SOCE by IP3R requires IP3 binding but not a functional pPore in SH-SY5Y cells.
(A) SOCE is activated when loss of Ca2+ from the ER through IP3Rs activates STIM1 (i). Our results suggest an additional role for IP3Rs (ii). (B) SH-SY5Y cells expressing IP3R1-shRNA alone or with IP3R1 or IP3R1DA were stimulated with thapsigargin (Tg, 1 µM) in Ca2+-free HBSS before restoring extracellular Ca2+ (2 mM). Traces show mean ± s.e.m, for 100–150 cells from three experiments. (C) Cells expressing IP3R1-shRNA and IP3R1DA were treated with NS-siRNA or Orai1-siRNA before measuring Tg-evoked Ca2+ entry. Traces show mean ± s.e.m. for 85–100 cells from three experiments. (D) Summary results (mean ± s.e.m.) show peak increases in [Ca2+]c (Δ[Ca2+]c) evoked by Ca2+ restoration. (E) Tg-evoked Ca2+ entry in cells expressing IP3R1-shRNA with IP3R1, IP3R1RQ or IP3R1RQ/KQ. Traces show mean ± s.e.m, for 90–150 cells from three experiments. (F) Summary results (mean ± s.e.m.) show peak increases in [Ca2+]c (Δ[Ca2+]c) evoked by Ca2+ restoration. Different letter codes (panels D, F) indicate significantly different values, p<0.001, for multiple comparison one-way ANOVA and pair-wise Tukey’s test and for two genotype comparison Mann Whitney U-test. See also Figure 3—figure supplement 1—source data 1. Source data in Figure 3—source data 1.
-
Figure 3—source data 1
Regulation of SOCE by IP3R requires IP3 binding but not a functional pPore in SH-SY5Y cells.
- https://cdn.elifesciences.org/articles/80447/elife-80447-fig3-data1-v2.zip
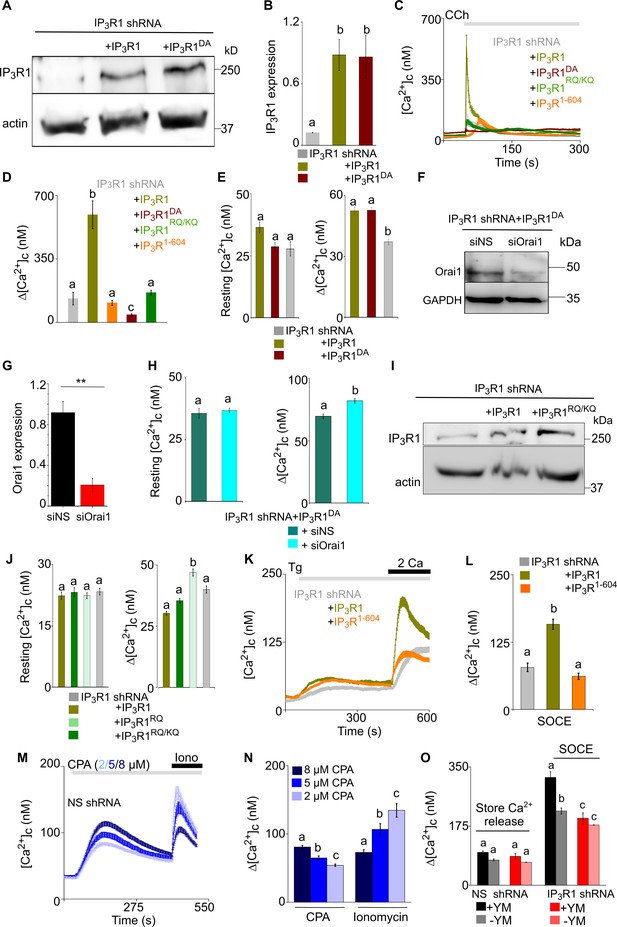
Attenuated SOCE in SH-SY5Y cells lacking IP3R1 is rescued by expression of pore-dead IP3R1 with a functional IP3-binding site.
(A) WB with IP3R1 antibody showing expression of IP3R1 and IP3R1DA in SH-SY5Y cells expressing IP3R1-shRNA. (B) Summary (mean ± s.e.m, n=3) shows IP3R1 expression normalized to actin. p<0.001, one-way ANOVA with pair-wise Tukey’s test. Here, and in most subsequent panels, different letters indicate groups that are statistically different, with the test defined for each panel. (C) Ca2+ signals evoked by carbachol (CCh 3 μM) in SH-SY5Y cells expressing IP3R1-shRNA alone or with IP3R1, IP3R1DA, IP3R1RQ/KQ or IP3R11-604. Traces show mean ± s.e.m. from three experiments. (D) Summary results (mean ± s.e.m) show peak changes in [Ca2+]c evoked by CCh (Δ[Ca2+]c). p<0.001, one-way ANOVA with pair-wise Tukey’s test. (E) Resting [Ca2+]c (left) and thapsigargin (Tg)-evoked Ca2+ release (1 µM, Δ[Ca2+]c) (right) in cells treated as indicated. p<0.001, one-way ANOVA with pair-wise Tukey’s test. (F) WB showing Orai1 expression in cells expressing IP3R1-shRNA and IP3R1DA, and then treated with NS or Orai1-siRNA. (G) Summary results (normalized to GAPDH expression, mean ± s.d, n=3). **p<0.01, Student’s t-test with unequal variances. (H) Resting [Ca2+]c (left) and Tg-evoked Ca2+ release (right) in cells expressing IP3R1-shRNA and IP3R1DA, and then treated with NS or Orai1-siRNA. p<0.05, Mann-Whitney U-test. (I) WB for IP3R1 in cells expressing IP3R1-shRNA alone or with IP3R1 or IP3R1RQ/KQ. Results typical of two independent experiments. (J) Resting [Ca2+]c (left) and Ca2+ release evoked by Tg (right) in cells expressing IP3R1-shRNA alone or with the indicated IP3R1. Mean ± s.e.m., n=3 experiments. p<0.001, one-way ANOVA with pair-wise Tukey’s test. (K) Changes in [Ca2+]c evoked by Tg (10 µM) in Ca2+-free HBSS and then after restoration of extracellular Ca2+ (2 mM) in cells expressing IP3R1-shRNA alone or with IP3R1 or IP3R11-604. Mean ± s.e.m. from three experiments with >100 cells. (L) Summary results show peak increases in [Ca2+]c evoked by Ca2+ restoration in the indicated cells. p<0.001, one-way ANOVA with pair-wise Tukey’s test. (M) SH-SY5Y cells expressing NS-shRNA were stimulated with the indicated concentrations of CPA in Ca2+-free HBSS to partially deplete ER Ca2+ stores and then with ionomycin (Iono, 1 µM) to release all remaining Ca2+. Results show mean ± s.e.m. for >30 cells from three experiments. (N) Summary results (mean ± s.e.m.) show Δ[Ca2+]c evoked by CPA or ionomycin. p<0.001, one-way ANOVA with pair-wise Tukey’s test. The results confirm that the lower concentrations of CPA partially deplete the ER of Ca2+. (O) Summary results (mean ± s.e.m, 80–100 cells from three experiments) show the peak increases in [Ca2+]c (Δ[Ca2+]c) after adding Tg (store Ca2+ release) and after restoring extracellular Ca2+ (SOCE) with or without YM-254890 treatment. Different letter codes indicate significantly different values within the Ca2+ release or SOCE groups, p<0.001, one-way ANOVA and pair-wise Tukey’s test. Source data in Figure 3—figure supplement 1—source data 1.
-
Figure 3—figure supplement 1—source data 1
Attenuated SOCE in SH-SY5Y cells lacking IP3R1 is rescued by expression of pore-dead IP3R1 with a functional IP3-binding site.
- https://cdn.elifesciences.org/articles/80447/elife-80447-fig3-figsupp1-data1-v2.zip
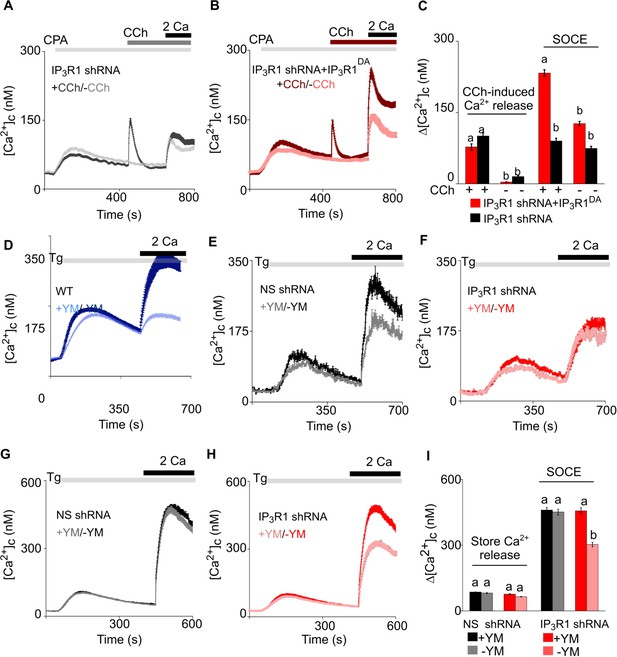
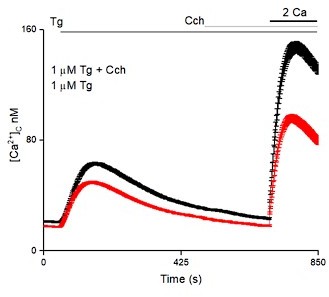
Receptor-regulated IP3 production stimulates SOCE in cells with empty Ca2+ stores and expressing pore-dead IP3R.
(A, B) SH-SY5Y cells expressing IP3R1-shRNA alone (A) or with IP3R1DA (B) were treated with a low concentration of CPA (2 µM) in Ca2+-free HBSS to partially deplete the ER of Ca2+ and sub-maximally activate SOCE (see Figure 3—figure supplement 1M–N). Carbachol (CCh, 1 µM) was then added to stimulate IP3 formation through muscarinic receptors, and extracellular Ca2+ (2 mM) was then restored. Traces (mean ± s.e.m of 68–130 cells from three experiments) show responses with and without the CCh addition. (C) Summary results show the peak increases in [Ca2+]c (Δ[Ca2+]c) after addition of CCh (CCh-induced Ca2+ release) and then after restoring extracellular Ca2+ (SOCE). (D–F) SH-SY5Y cells wild type (WT) (D) and expressing NS-shRNA (E) or IP3R1-shRNA (F) were treated with YM-254890 (YM, 1 µM, 5 min) in Ca2+-free HBSS to inhibit Gαq and then with thapsigargin (Tg, 1 µM) before restoring extracellular Ca2+ (2 mM). Traces show mean ± s.e.m of ~120 cells from three experiments. (G–I) Similar analyses of HEK cells. Summary results (mean ± s.e.m, 50–100 cells from three experiments) are shown in (I). Different letter codes (panels C and I) indicate significantly different values within the store Ca2+ release or SOCE groups, p<0.001, one-way ANOVA and pair-wise Tukey’s test. See also Figure 4—figure supplement 1. Source data in Figure 4—source data 1.
-
Figure 4—source data 1
Receptor-regulated IP3 production stimulates SOCE in cells with empty Ca2+ stores and expressing pore-dead IP3R.
- https://cdn.elifesciences.org/articles/80447/elife-80447-fig4-data1-v2.zip
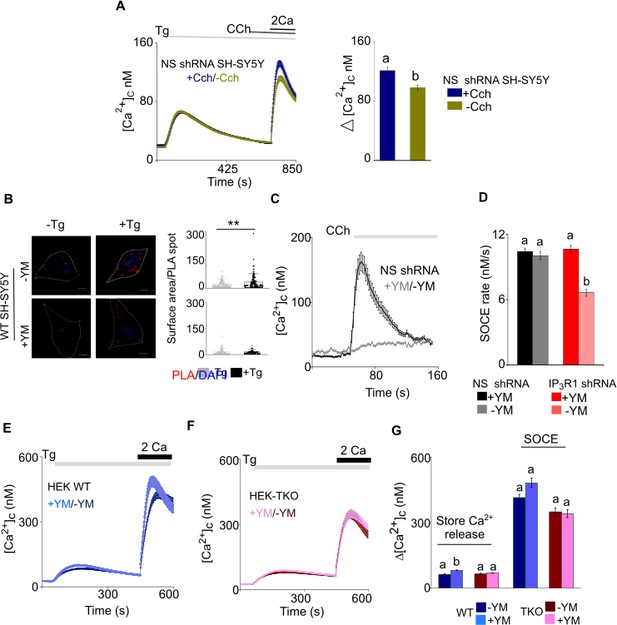
Effects of generating IP3 and inhibiting Gq on Ca2+ signals and STIM1-Orai1 interactions in SH-SY5Y and HEK cells.
(A) SOCE is enhanced in SH-SY5Y cells expressing control-shRNA after ER-Ca2 depletion (thapsigargin, 2 µM) followed by carbachol (CCh, 1 µM) stimulated IP3 formation. Traces (mean ± s.e.m of ~270 cells each from five experiments) show responses with and without the CCh addition. Summary results show the peak increases in [Ca2+]c (Δ[Ca2+]c) for SOCE after restoring extracellular Ca2+. Different letters indicate significantly different values of p<0.001 by Mann- Whitney U-test. (B) PLA analyses of interactions between STIM1 and Orai1 in wild type (WT) SH-SY5Y cells untreated (-YM) or treated (+YM, 1 μm) with Gq inhibitor YM-254890. Confocal images are shown for control cells (-Tg) or after treatment with thapsigargin (+Tg, 1 µM) in Ca2+-free HBSS. PLA reaction product is red, and nuclei are stained with DAPI (blue). Scale bars, 5 µm. Summary results show the surface area of the PLA spots for ~20 cells from two independent analyses. Individual values, median (bar) and 25th and 75th percentiles (box). **p<0.01, Student’s t-test with unequal variances. (C) HEK cells expressing NS shRNA and treated with the Gq inhibitor, YM-254890 (YM, 1 μM, 5 min) were stimulated with carbachol (CCh, 100 μM). Traces show mean ± s.e.m. from three experiments with 30–40 cells. The results confirm that treatment with YM-254890 effectively uncouples CCh from IP3-evoked Ca2+ signals. (D) From results shown in Figure 4G and H, rates of thapsigargin-evoked Ca2+ entry were determined. Summary shows mean ± s.e.m, from 100 to 120 cells. p<0.001, Mann-Whitney U-test. (E–F) Changes in [Ca2+]c evoked by thapsigargin (Tg, 1 μM) in Ca2+-free HBSS and then after restoration of extracellular Ca2+ (2 mM) in WT (C) and TKO (D) HEK cells after treatment with YM-254890 (YM, 1 μM, 5 min). Traces show mean ± s.e.m. from three experiments with 100–120 cells. (G) Summary results show peak increase in [Ca2+]c (Δ[Ca2+]c) evoked by addition of thapsigargin and then after restoring extracellular Ca2+ (SOCE). Mean ± s.e.m. Different letter codes indicate significantly different values, p<0.001, Mann- Whitney U-test. Source data in Figure 4—figure supplement 1—source data 1.
-
Figure 4—figure supplement 1—source data 1
Effects of generating IP3 and inhibiting Gq on Ca2+ signals and STIM1-Orai1 interactions in SH-SY5Y and HEK cells.
- https://cdn.elifesciences.org/articles/80447/elife-80447-fig4-figsupp1-data1-v2.zip
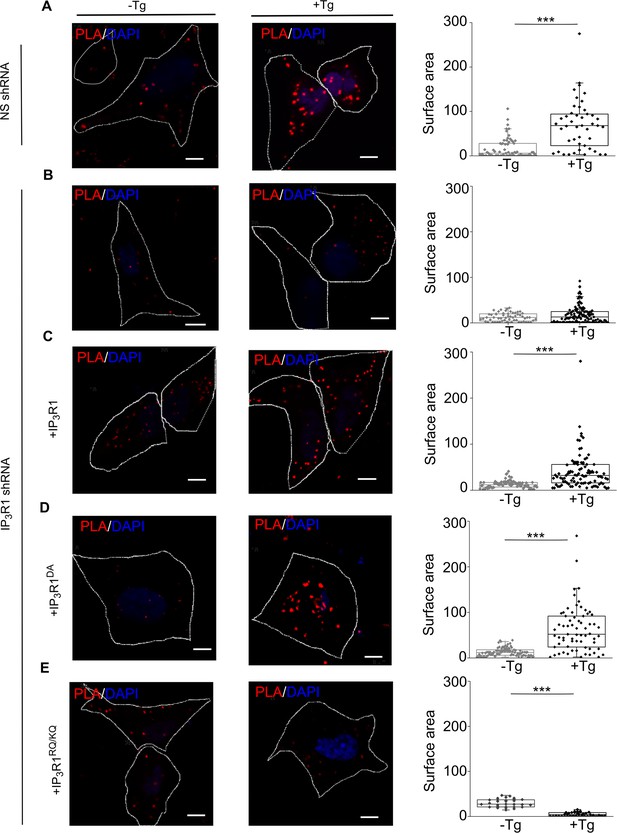
IP3Rs promote interaction of STIM1 with Orai1.
(A–E) PLA analyses of interactions between STIM1 and Orai1 in SH-SY5Y cells expressing NS-shRNA (A) or IP3R1-shRNA alone (B) or with IP3R1 (C), IP3R1DA (D) or IP3R1RQ/KQ (E). Confocal images are shown for control cells or after treatment with thapsigargin (Tg, 1 µM) in Ca2+-free HBSS. PLA reaction product is red, and nuclei are stained with DAPI (blue). Scale bars, 5 µm. Summary results show the surface area of the PLA spots for 8–10 cells from two independent analyses. Individual values, median (bar) and 25th and 75th percentiles (box). ***p < 0.001, Student’s t-test with unequal variances. See also Figure 5—figure supplement 1. Source data in Figure 5—source data 1.
-
Figure 5—source data 1
IP3Rs promote interaction of STIM1 with Orai1.
- https://cdn.elifesciences.org/articles/80447/elife-80447-fig5-data1-v2.zip
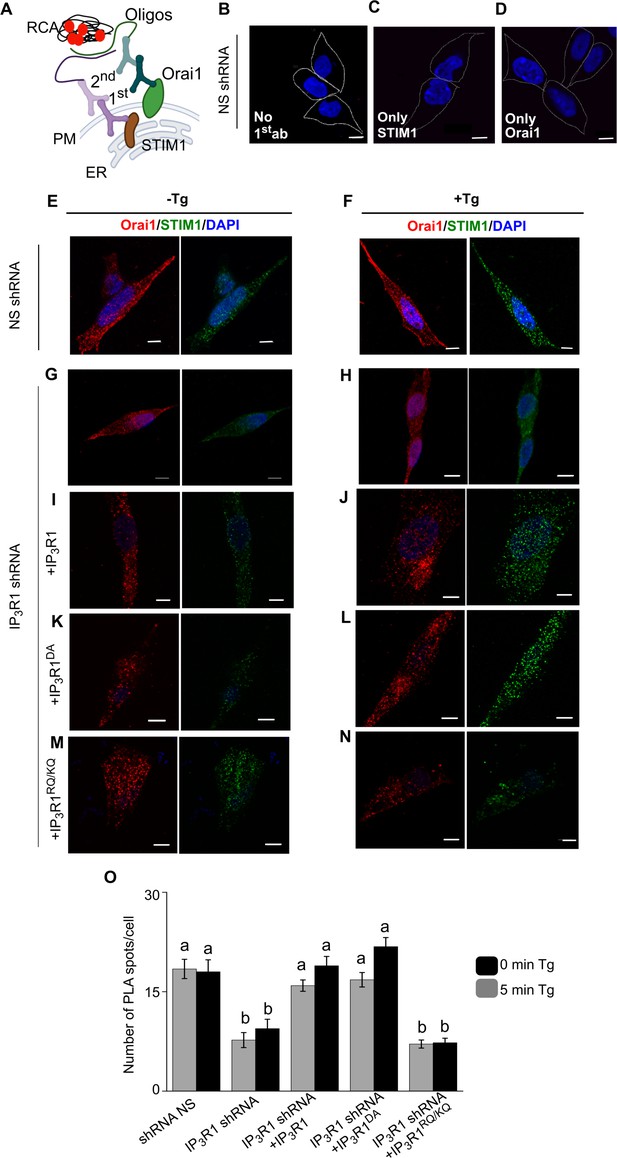
Validation of PLA measurements of Orai1-STIM1 interactions.
(A) PLA using antibodies to Orai1 and STIM1 to establish whether they are associated. Complementary oligonucleotides conjugated to a secondary antibody (2nd) targeting their respective primary antibodies (1st) hybridize when they are close to each other, allowing rolling circle amplification (RCA) and production of a red fluorescent product. The latter can be quantified from the surface area of the fluorescent spots or their intensity. We obtained indistinguishable results with both methods of quantification. We therefore show only results obtained from surface-area measurements. (B–D) Confocal images from PLA assays of SH-SY5Y cells expressing NS-shRNA performed with no primary antibody (B), or with primary antibody for only STIM1 (C) or only Orai1 (D). Scale bars, 5 µm. (E, F) Confocal images of SH-SY5Y cells expressing NS-shRNA with (F) or without thapsigargin treatment (E) (Tg, 1 µM, in Ca2+-free HBSS). Cells are stained with DAPI and immunostained for Orai1 or STIM1. Scale bars, 5 µm. (G–N) Similar analyses of cells expressing IP3R1-shRNA alone (G, H), or with IP3R1 (I, J), IP3R1DA (K, L) or IP3R1RQ/KQ (M, N). Results (E–N) are typical of two experiments. (O) Number of PLA spots per cell in the indicated genotypes with (5 min) or without (0 min) thapsigargin treatment (Tg, 1 µM in Ca2+-free HBSS) in the indicated genotypes. Different letter indicates significantly different values, p<0.001 one-way ANOVA and pair-wise Tukey’s test. The PLA results are shown in Figure 5. Source data in Figure 5—figure supplement 1—source data 1.
-
Figure 5—figure supplement 1—source data 1
Validation of PLA measurements of Orai1-STIM1 interactions.
- https://cdn.elifesciences.org/articles/80447/elife-80447-fig5-figsupp1-data1-v2.zip

Ligand-bound IP3R1 supports SOCE-dependent STIM1 movement to ER-PM contact sites.
(A–B) Representative TIRF images of mVenus STIM1 co-transfected with either wild type mcherry-rat IP3R1 (A) or IP3R1RQ/KQ (ligand binding mutant), (B) in wild type SH-SY5Y cells before (Basal) and after CPA induced store depletion (CPA treated) at 4 min and 7 min. On the right are shown RGB profile plots of STIM1 (green) and IP3R1, wild type or mutant (magenta) corresponding to the rectangular selections (Cell 1 and Cell 2). Scale bar is 10 µm.(C–D) Changes in number of IP3R1 (C) and STIM1 (D) puncta upon CPA-induced store depletion over a period of 10 min in the indicated genotypes. Mean ± s.e.m from seven cells from n=6 independent experiments. (E) Summary result (mean ± s.e.m) showing the change in the number of maximum STIM1 puncta formed after CPA-induced store depletion in the indicated genotypes. Mean ± s.e.m. of seven cells from n=6 independent experiments. Different letters indicate significant differences, p<0.05, Mann-Whitney U-test. See also Figure 6—figure supplement 1. Source data in Figure 6—source data 1.
-
Figure 6—source data 1
Ligand-bound IP3R1 supports SOCE-dependent STIM1 movement to ER-PM contact sites.
- https://cdn.elifesciences.org/articles/80447/elife-80447-fig6-data1-v2.zip
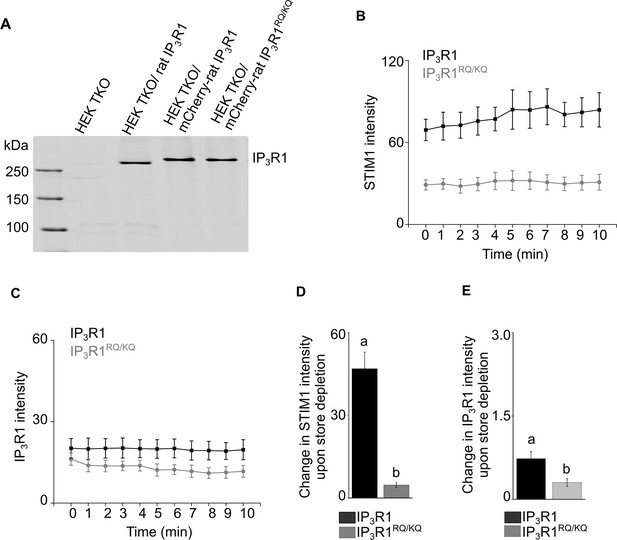
Validation of fluorescent-tagged rat IP3R1 constructs.
(A) Western blot image showing the expression of IP3R1 in the indicated genotypes Results with these constructs are shown in Figure 6. (B–C) Changes in the intensity of STIM1 and IP3R1 puncta (>2 pixel) upon CPA-induced store depletion over a period of 10 min in the indicated genotypes. Intensity changes were measured within ROIs drawn in each cell, where new STIM1 puncta formed visibly upon CPA-induced store depletion. Mean ± s.e.m from seven cells from n=6 independent experiments. (D) Summary result (mean ± s.e.m) showing the change in intensity of STIM1 after CPA-induced store depletion in the indicated genotypes. Mean ± s.e.m. of seven cells from n=6 independent experiments. Different letters indicate significant differences, p<0.05, Mann-Whitney U-test. (E) Summary result (mean ± s.e.m) showing the change in the intensity of IP3R1 after CPA-induced store depletion in the indicated genotypes. Mean ± s.e.m. of seven cells from n=6 independent experiments. Different letters indicate significant differences, p<0.05, Student’s t-test with unequal variances. Source data in Figure 6—figure supplement 1—source data 1.
-
Figure 6—figure supplement 1—source data 1
Validation of fluorescent-tagged rat IP3R1 constructs.
- https://cdn.elifesciences.org/articles/80447/elife-80447-fig6-figsupp1-data1-v2.zip

Extended synaptotagmins rescue SOCE in cells lacking IP3R1.
(A) SH-SY5Y cells expressing IP3R1-shRNA alone or with E-Syt1 were stimulated with Tg (1 µM) in Ca2+-free HBSS before restoring extracellular Ca2+ (2 mM). Traces show mean ± s.e.m, for 20–80 cells from three experiments. (B) Summary results show Δ[Ca2+]c evoked by restoring Ca2+ (SOCE). Mean ± s.e.m, ***p < 0.001, Mann-Whitney U- test. (C) Summary results (mean ± s.e.m, n=20–80 cells) show resting [Ca2+]c (left) and the peak Ca2+ signals (Δ[Ca2+]c) evoked by thapsigargin (Tg, 1 µM) in Ca2+-free HBSS for SH-SY5Y cells expressing IP3R1-shRNA alone or with human E-Syt1. (D) Cells over-expressing E-Syt1 and treated with IP3R1-shRNA in combination with either NS or STIM1 siRNA were stimulated with Tg (1 µM) in Ca2+-free HBSS before restoration of extracellular Ca2+ (2 mM). Mean ± s.e.m. from three experiments with 30–40 cells. (E, F) Summary results (mean ± s.e.m, n=30–40 cells) show SOCE evoked by Tg (E), resting [Ca2+]c and the Tg-evoked Ca2+ release from intracellular stores (F). ***p< 0.001, Mann-Whitney U- test. (G) Similar analyses of cells expressing NS shRNA alone or with human E-Syt1 and then treated with Tg (1 µM) in Ca2+-free HBSS before restoring extracellular Ca2+ (2 mM). Mean ± s.e.m. from three experiments with 115–135 cells. (H, I) Summary results (mean ± s.e.m, n=115–135 cells) show resting [Ca2+]c (H) and Δ[Ca2+]c evoked by Tg (store release) or Ca2+ restoration (SOCE) (I). No significant difference, Mann Whitney U-test. Source data in Figure 7—source data 1.
-
Figure 7—source data 1
Extended synaptotagmins rescue SOCE in cells lacking IP3R1.
- https://cdn.elifesciences.org/articles/80447/elife-80447-fig7-data1-v2.zip
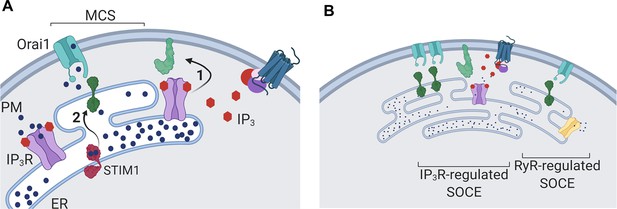
Dual regulation of SOCE by IP3Rs.
(A) SOCE is activated when loss of Ca2+ from the ER, usually mediated by opening of IP3Rs when they bind IP3, causes STIM to unfurl cytosolic domains (2). The exposed cytosolic domains of STIM1 reach across a narrow gap between the ER and PM at a MCS to interact with PIP2 and Orai1 in the PM. Binding of STIM1 to Orai1 causes pore opening, and SOCE then occurs through the open Orai1 channel. We show that IP3Rs when they bind IP3 also facilitate interactions between Orai1 and STIM, perhaps by stabilizing the MCS (1). Receptors that stimulate IP3 formation thereby promote both activation of STIM (by emptying Ca2+ stores) and independently promote interaction of active STIM1 with Orai1. (B) Other mechanisms, including ryanodine receptors (RyR), can also release Ca2+ from the ER. We suggest that convergent regulation of SOCE by IP3R with bound IP3 allows receptors that stimulate IP3 formation to selectively control SOCE.
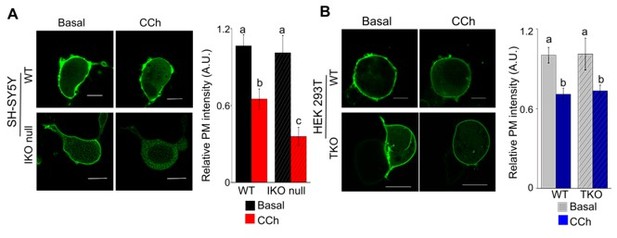
Differential PIP2 dynamics in neuronal and non-neuronal cells.
(A-B) Confocal images of cells expressing a PIP2 biosensor PH-PLCD1-GFP. SH-SY5Y (WT and IKO null) (A) and HEK- (WT and TKO) cells (B) before (basal) and after carbachol treatment (CCh, 100μM). Scale bar is 10µm. Summary results (mean+ s.e.m, from 3 independent experiments with a total of 15-18 cells) show the relative intensity of plasma membrane (PM) bound PH-PLCD1-GFP normalized to total fluorescence in their respective WT cells. Different alphabet indicate P<0.01, Student’s t-test with unequal variances.
Additional files
-
MDAR checklist
- https://cdn.elifesciences.org/articles/80447/elife-80447-mdarchecklist1-v2.docx
-
Supplementary file 1
Details of plasmids and recombinant DNAs.
- https://cdn.elifesciences.org/articles/80447/elife-80447-supp1-v2.docx