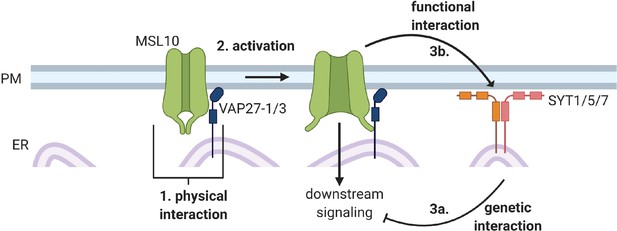
Unbiased proteomic and forward genetic screens reveal that mechanosensitive ion channel MSL10 functions at ER–plasma membrane contact sites in Arabidopsis thaliana
Figures
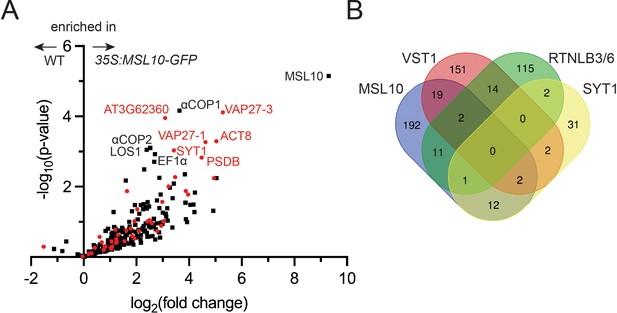
Co-immunoprecipitation–liquid chromatography-tandem mass spectrometry (LC-MS/MS) identifies the MSL10-GFP interactome, which shares similarities to previous endoplasmic reticulum–plasma membrane contact site (EPCS) interactomes.
(A) Volcano plot showing the abundance of proteins detected in immunoprecipitations of MSL10-GFP in 35S:MSL10-GFP seedlings (right) compared to those identified in mock immunoprecipitations using WT Col-0 seedlings (left). Proteins were identified by LC-MS/MS, and the average abundance of each was quantified from the MS1 precursor ion intensities. Only those proteins with at least eight peptide spectral matches are shown. Each protein is plotted based on its -log10(p-value) of significance based on four biological replicates relative to its log2(fold change) of abundance (35S:MSL10-GFP/ WT). Proteins also detected in immunoprecipitations with the EPCS proteins SYT1 (Ishikawa et al., 2020), VST1 (dataset filtered for proteins with >8 peptide-spectral matches [PSMs]; Ho et al., 2016), and VAP27-1/3 (Stefano et al., 2018) or plasmodesmata-associated RTNLB3/6 (Kriechbaumer et al., 2015) are represented as red circles; proteins unique to the MSL10 interactome are represented as black squares. The 11 most significantly enriched proteins are labeled (p-value<0.002). (B) The overlap of the indicated interactomes with that of MSL10. The VAP27-1/3 interactome (Stefano et al., 2018) was not included here because only eight selected interactors were reported.
-
Figure 1—source data 1
Peptide abundances from LC-MS/MS from mock, MSL10-GFP, and MSL10 7D-GFP immunoprecipitations.
- https://cdn.elifesciences.org/articles/80501/elife-80501-fig1-data1-v2.xlsx
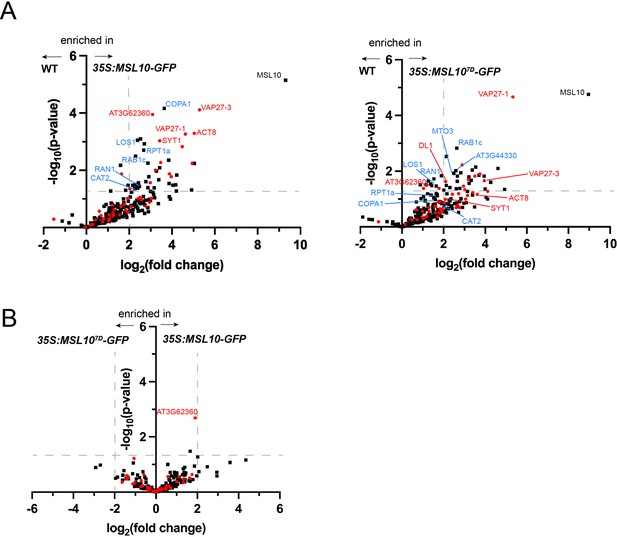
Similar proteins were identified in MSL10-GFP and MSL107D-GFP immunoprecipitations.
(A) Volcano plots showing the preferential abundance of proteins detected in immunoprecipitations of 35S:MSL10-GFP (left, reproduced from Figure 1A) or 35S:MSL107D-GFP (right) compared to those of mock immunoprecipitations of WT Col-0 seedlings. (B) displays the relative abundance of proteins in 35S:MSL10-GFP (right) vs. 35S:MSL107D-GFP (left) immunoprecipitations. Proteins were identified by liquid chromatography-tandem mass spectrometry (LC-MS/MS), and the average abundance of each was quantified from the MS1 precursor ion intensities, and only those proteins with at least eight peptide spectral matches are shown. Each protein is plotted based on its -log10(p-value) of significance based on four biological replicates relative to its log2(fold change) of abundance. Data points indicated as red circles have previously detected in interactomes of SYT1 (Ishikawa et al., 2020), VAP27-1/3 (Stefano et al., 2018), RTNLB3/6 (Kriechbaumer et al., 2015), and VST1 (Ho et al., 2016). Labeled are proteins that were selected for further testing in Figure 2A, selected because in either the MSL10-GFP and/or MSL107D-GFP co-immunoprecipitations they were above the cutoffs indicated as dashed gray lines: fold change > 4 and p-values<0.05. Those with red labels have been found previously in endoplasmic reticulum–plasma membrane contact site (EPCS) or plasmodesmatal interactomes, and those in blue have not.
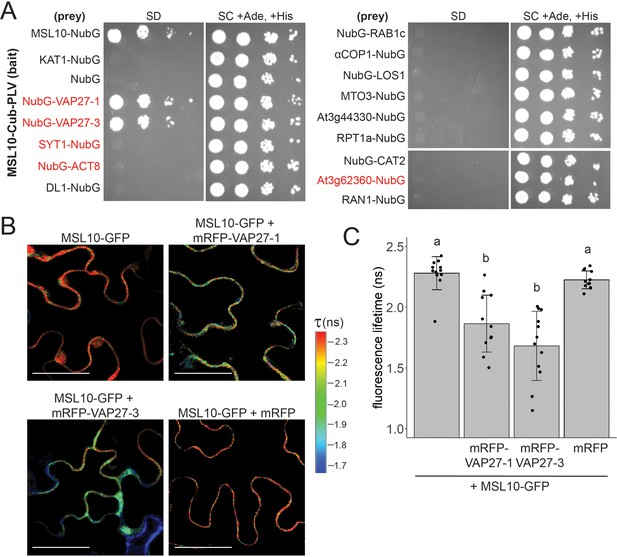
MSL10 interacts with VAP27-1 and VAP27-3.
(A) Mating-based split-ubiquitin (mbSUS) assay. VAMP-associated protein 27-1 (VAP27-1), VAP27-3, synaptotagmin 1 (SYT1), actin 8 (ACT8), dynamin-like (DL1), RAB GTPase homolog 1c (RAB1c), coatomer α1 subunit (αCOP1), LOW EXPRESSION OF OSMOTICALLY RESPONSIVE GENES 1 (LOS1), METHIONINE OVERACCULATOR 3 (MTO3), AT3G44330, regulatory particle triple-A 1A (RPT1a), catalase 2 (CAT2), AT3G62360, and Ras-related nuclear protein 1 (RAN1) were fused to NubG and tested for interaction with Cub-tagged MSL10. Proteins labeled in red were previously detected at endoplasmic reticulum–plasma membrane contact sites (EPCSs). The results in (A) are consistent with a second independent mbSUS assay using independent transformants. (B, C) In vivo Förster resonance energy transfer–fluorescence lifetime imaging microscopy (FRET-FLIM) on UBQ:MSL10-GFP and UBQ:mRFP-VAP27-1 or UBQ:mRFP-VAP27-3 transiently expressed in tobacco. (B) Representative heat maps of the fluorescence lifetime (τ) of GFP measured in tobacco abaxial epidermal cells 5 days post-infiltration. Scale = 50 µm. (C) Average GFP fluorescence lifetime. Each data point represents the value from one field of view (three fields of view per plant from four infiltrated plants for a total of n = 12 for each combination). Error bars, SD. Groups indicated by the same letter are not statistically different according to ANOVA with Tukey’s post-hoc test.
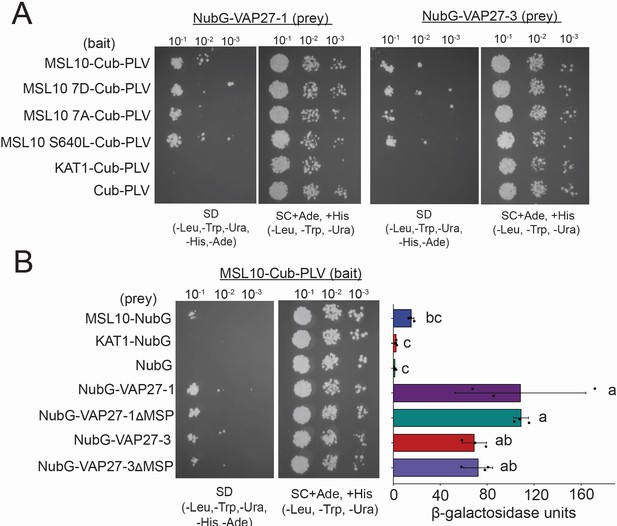
MSL10 signaling mutants interact with VAP27-1 and VAP27-3, and the VAP27 MSP domain is dispensable for interaction.
Mating-based split-ubiquitin assays testing (A) the interaction of full-length VAP27-1 and VAP27-3 with mutant versions of full-length MSL10 and (B) the interaction of full-length MSL10 with variants of VAP27-1 and VAP27-3 that lacked their major sperm protein (MSP) domain (VAP27-1∆6-125 and VAP27-3∆23-142). MSL107A is a phosphodead variant in which the seven phosphoserines in the N-terminus of MSL10 are mutated to alanine (Veley et al., 2014; Basu et al., 2020b). The MSL10S640L substitution (msl10-3G allele) occurs in the cytosolic C-terminal domain of MSL10 (Zou et al., 2016). Both variants trigger constitutive overactivation of MSL10 cell death signaling. At right in (B): measurements of β-galactosidase activity in a liquid-based assay testing the same combinations at left using CPRG as substrate.
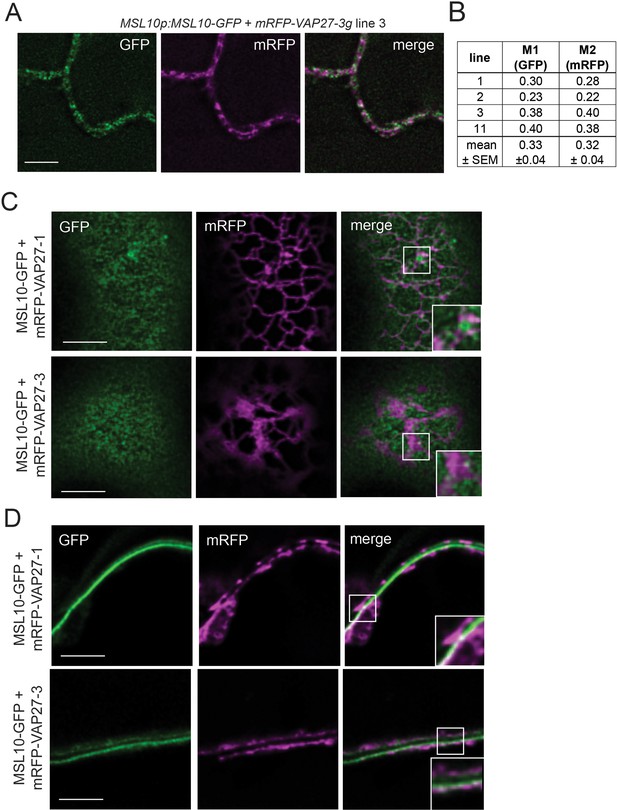
A subpopulation of MSL10 co-localizes with a subpopulation of VAP27-1 and VAP27-3.
(A) Equatorial deconvolved confocal laser scanning micrographs of leaf abaxial epidermal cells from stable Arabidopsis T1 lines co-expressing MSL10-GFP and mRFP-VAP27-3 driven by their endogenous promoters. Scale = 5 µm. (B) Mander’s overlap coefficients M1 and M2 calculated from images taken from four independent T1 lines. (C, D) Deconvolved confocal micrographs showing a Z-slice at the top (cortical, C) and the middle (equatorial, D) of tobacco epidermal cells transiently expressing UBQ:MSL10-GFP and UBQ:mRFP-VAP27-1 or UBQ:mRFP-VAP27-3. Images were taken 5 days after infiltration. Scale = 5 µm.
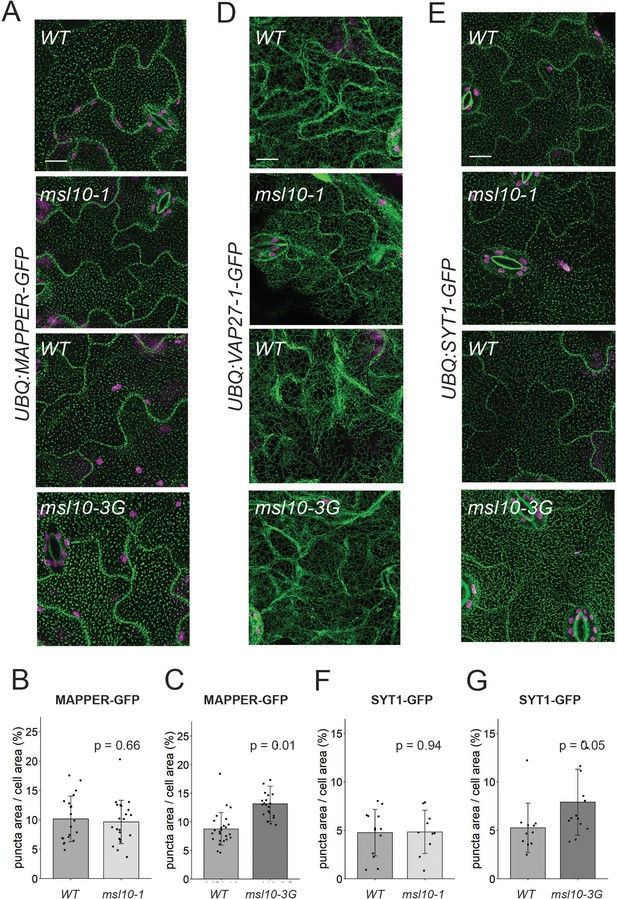
Some endoplasmic reticulum–plasma membrane contact sites (EPCSs) are expanded in msl10-3G plants.
Confocal Z-projections (maximum intensity projection of Z-slices from the top to the middle of cells) of GFP-tagged proteins in the indicated MSL10 backgrounds. MAPPER-GFP (A), VAP27-1-GFP (D), and SYT1-GFP (E) in 4-week-old abaxial leaf epidermal cells. Plants shown here are cousins (A, E) or siblings (D). Green, GFP; magenta, chlorophyll autofluorescence. Scale = 10 µm. Quantification of the percentage of the leaf epidermal cell volume taken up by MAPPER-GFP (B, C) or SYT1-GFP (F, G) puncta in plants in the msl10-1 or msl10-3G background compared to WT cousins. Each data point represents a biological replicate: the mean value of 20–50 epidermal cells from one plant, n = 10–25 plants per genotype from two or three separately grown flats. Error bars, SD. Means were compared by Student’s t-tests when data was normally distributed (B, F) or Mann–Whitney U-tests when it was not (C, G).
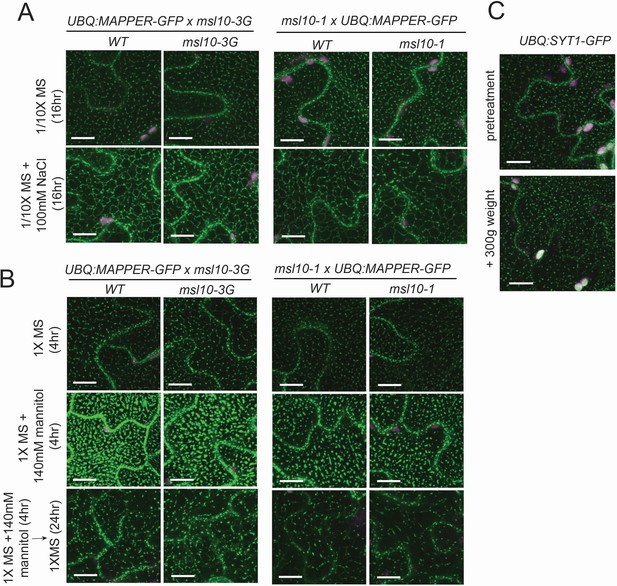
MSL10 does not influence rearrangements in endoplasmic reticulum–plasma membrane contact sites (EPCS) morphology in response to osmotic stress in seedlings.
Confocal maximum intensity Z-projections of cotyledon epidermal cells. Green, GFP; magenta, chlorophyll autofluorescence. Scale = 10 µm. The seedlings being compared in (A, B) are F3 cousins. (A) Five-day-old seedlings were transferred from plates and incubated for 16 hr in liquid 1/10× MS or 1/10× MS + 100 mM NaCl. (B) Seedlings were grown on 1× MS or 1× MS +140 mM mannitol plates for 5 days. Seedlings were transferred to liquid media of the same concentration supplemented with 600 nM isoxaben and allowed to equilibrate for 4 hr before imaging (as described in Basu and Haswell, 2020a). MAPPER-GFP puncta size is larger in the presence of mannitol, in contrast to observations by Lee et al., 2019, and the difference might be attributable to the presence of mannitol in the plates for the entire life of the seedlings, used here. A subset of seedlings incubating in 1× MS + 140 mM mannitol + 600 nM isoxaben were transferred to liquid 1× MS + 600 nM isoxaben to trigger cell swelling, and these were imaged 24 hr later. (C) Five-day-old seedlings stably expressing UBQ:SYT1-GFP were mounted in water. Cotyledons were imaged before and after a 300 g weight was applied to a 22 × 22 mm coverslip for 20 s.

A forward genetic screen identified sdm26 and sdm34, dominant suppressors of msl10-3G height and ectopic cell death phenotypes.
(A) Schematic of the screen. (B) Images of the indicated plants after 4–5 weeks of growth. (C) Segregation of height phenotypes in the BC1F2 generation compared to the expected segregation ratio assuming the sdm alleles are dominant. (D) Siblings of backcrossed sdm26 and sdm34 mutants that were fixed for the sdm (suppressed dwarfing) or msl10-3G (dwarf) phenotypes. Top: 5-week-old BC1F2 plants of the indicated genotypes. Middle: 4-week-old BC1F3 progeny of plants at the top, as indicated with dashed lines. Bottom: leaves of 4-week-old BC1F3 plants stained with Trypan blue to assess cell death. These results are representative of at least five other plants for each genotype, in two separate experiments. Scale = 300 µm.
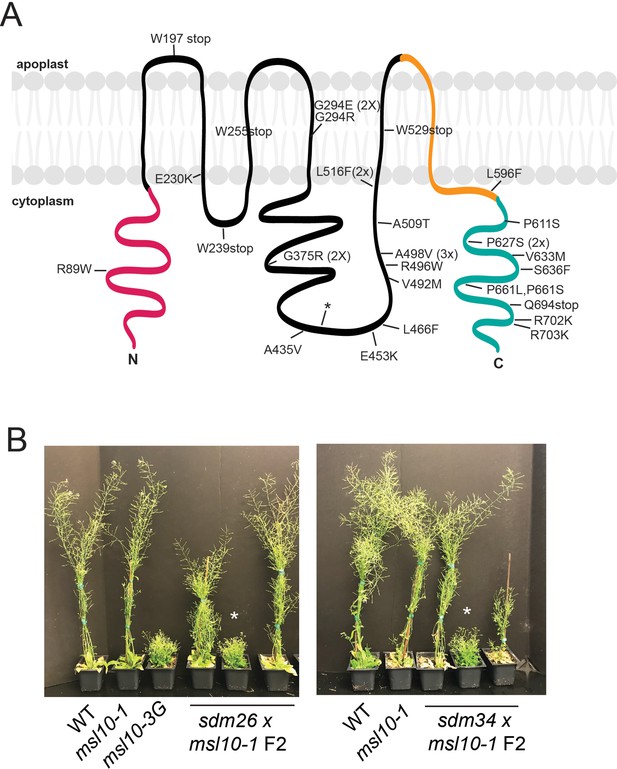
Intragenic sdm mutants and tests confirming that sdm26 and sdm34 causal mutations are extragenic.
(A) Intragenic sdm mutations mapped onto the predicted MSL10 topology. In pink is the cytosolic N-terminal domain, in teal is the cytoplasmic C-terminal domain, and in orange is the pore-lining MscS domain. The asterisk marks the location of one sdm mutation predicted to retain the intron between the first and second exons. (B) Pictures of 5-week-old F2 plants. From the sdm26 × msl10-1 cross, 1 out of 21 F2 plants screened had a dwarf msl10-3G phenotype, indicated with the asterisk. From the sdm34 × msl10-1 cross, there were 2 out of 23 F2 plants that had the dwarfed phenotype. Other F2 plants from both crosses had either a WT or intermediate height. That the msl10-3G (dwarf) phenotype could be recovered after crossing to the null msl10-1 line indicated that the sdm26 and sdm34 alleles were not linked to MSL10, confirming that they were extragenic suppressors.
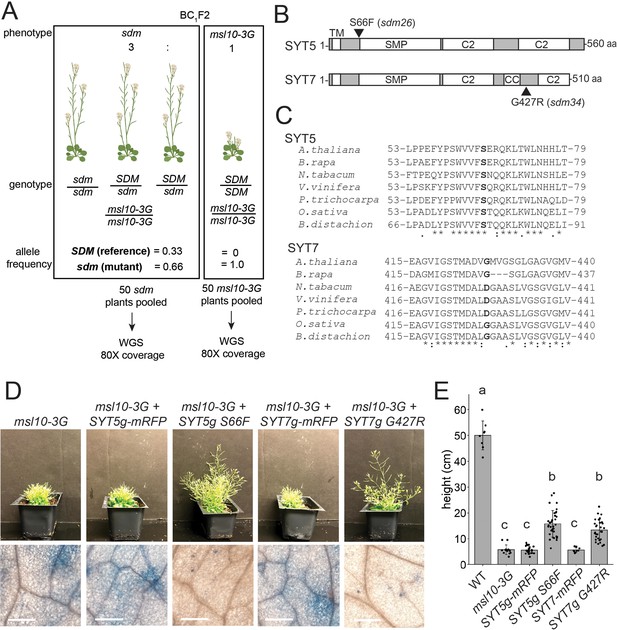
SYT5 S66F and SYT7 G427R are the causal mutations in sdm26 and sdm34, respectively.
(A) Overview of backcrossing and mapping-by-sequencing of sdm mutants. (B) Location of sdm26 and sdm34 missense mutations in the SYT5 and SYT7 proteins, respectively. UniProt was used to predict protein domains and their location. TM, transmembrane; SMP, synaptogamin-like mitochondrial-lipid-binding protein domain; CC, coiled coil; C2, Ca2+ binding. (C) Conservation of Ser66 and Gly427 residues in SYT5 and SYT7 homologs, respectively, in the predicted proteomes of selected angiosperms. (D, E) Phenotypes of msl10-3G plants expressing WT or sdm mutant SYT5 and SYT7 transgenes. (D) Top: images of representative T1 lines. Bottom: Trypan blue staining of a leaf from the same plants. Scale = 300 µm. (E) Mean and standard deviation of plant height of n = 9–32 T1 lines per construct, pooled from two similar experiments. Groups indicated with the same letters are not significantly different as assessed by ANOVA with Scheffe’s post-hoc test.
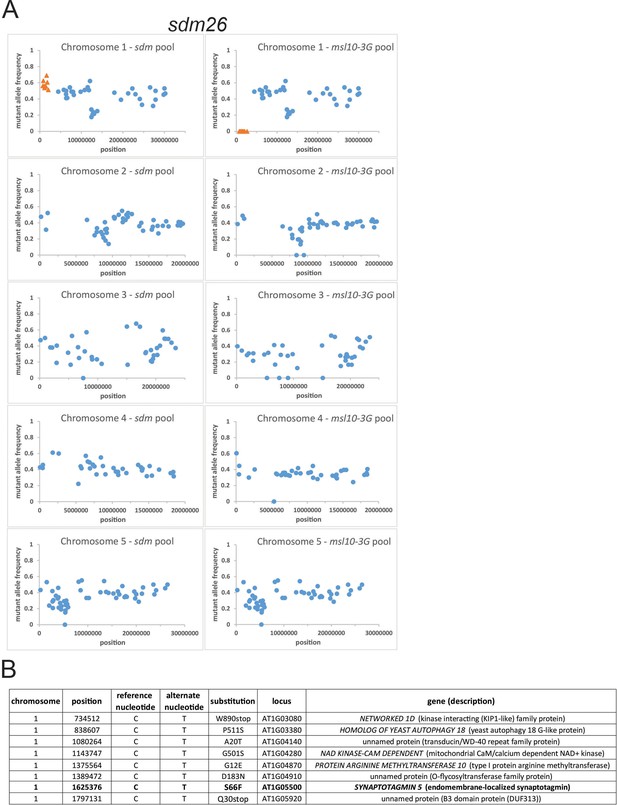
Mapping-by-sequencing reveals the chromosomal regions containing the causal mutations of sdm26.
sdm26 was backcrossed to msl10-3G plants, and the segregating BC1F2 population was pooled by phenotype and sent for whole-genome sequencing (WGS). Segregating BC1F2 populations were pooled by phenotype and sent for WGS. (A) For each SNP identified, the frequency at which this mutant nucleotide was detected compared to the reference nucleotide was calculated and plotted against its chromosomal position. Regions where SNPs are represented with orange triangles were predicted to contain the causal mutation as mutant alleles were absent in the msl10-3G phenotypic pool (dwarfed) and present in the sdm phenotypic pool (suppressed dwarfing) near the expected frequency of 0.66. (B) Details of the SNPs in the chromosomal intervals identified in (A). Gene names and functional descriptions were obtained from TAIR.

Mapping-by-sequencing reveals the chromosomal regions containing the causal mutations of sdm34.
sdm34 was backcrossed to msl10-3G plants, and the segregating BC1F2 population was pooled by phenotype and sent for whole-genome sequencing (WGS). Segregating BC1F2 populations were pooled by phenotype and sent for WGS. For each SNP identified, the frequency at which this mutant nucleotide was detected compared to the reference nucleotide was calculated and plotted against its chromosomal position. Regions where SNPs are represented with orange triangles were predicted to contain the causal mutation as mutant alleles were absent in the msl10-3G phenotypic pool (dwarfed) and present in the sdm phenotypic pool (suppressed dwarfing) near the expected frequency of 0.66. (B) Details of the SNPs in the chromosomal intervals identified in (A). Gene names and functional descriptions were obtained from TAIR.
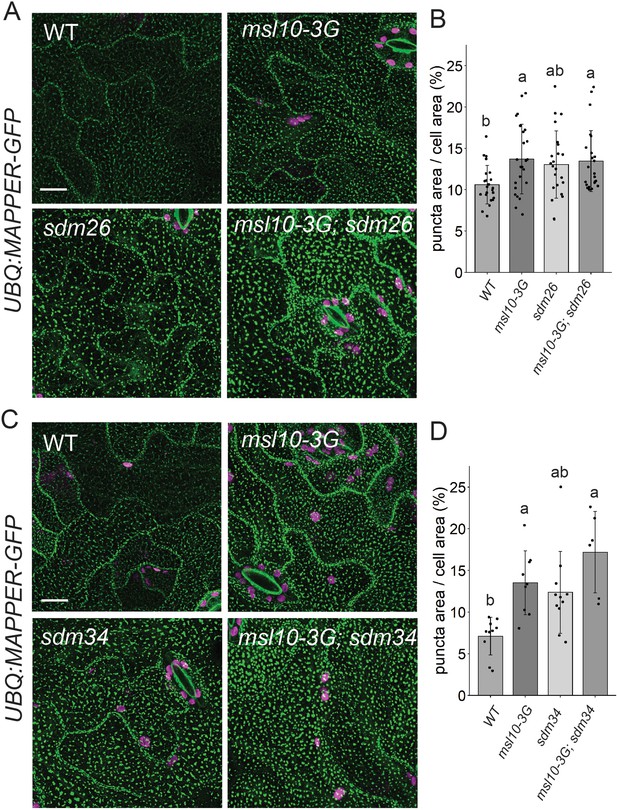
sdm26 and sdm34 alleles do not suppress expanded endoplasmic reticulum–plasma membrane contact sites (EPCSs) in msl10-3G leaves.
(A, C) Confocal Z-projections (maximum intensity projection of Z-slices from the top to the middle of cells) of MAPPER-GFP fluorescence in 4-week-old abaxial leaf epidermal cells of the indicated genotypes. Scale = 10 µm. (B, D) Quantification of the percentage of the leaf epidermal cell volume taken up by MAPPER-GFP puncta in plants of the indicated genotypes. Each data point represents a biological replicate (the mean value of 20–50 epidermal cells from one plant), n = 6–23 plants per genotype from three separately grown flats. Error bars, SD. Groups indicated with the same letters are not significantly different as assessed by Kruskal–Wallis with Dunn’s post-hoc test when measurements were not normally distributed (B) or ANOVA with Scheffe’s post-hoc test when they were (D).
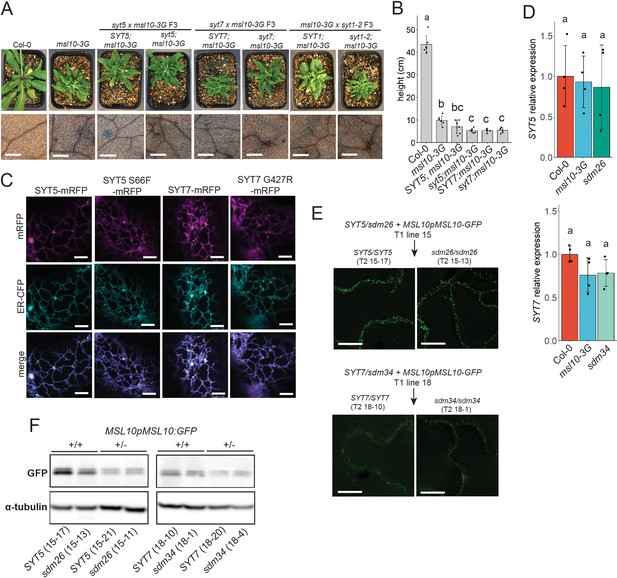
Null syt1, syt5, and syt7 alleles do not suppress msl10-3G phenotypes, and sdm26 and sdm34 mutations do not alter SYT5 or SYT7 localization, transcript levels, or MSL10 protein levels.
(A) Plants with the null syt5, syt7, or syt1-2 alleles were crossed to msl10-3G plants. Top row: shown are 4-week-old F3 cousins that are homozygous for the msl10-3G allele and homozygous for either the WT or null alleles. Bottom row: Trypan blue staining of 4-week-old leaves from the same plants. Scale = 300 µm. (B) Height of 6-week-old plants homozygous for both the msl10-3G allele and either WT or null SYT5 or SYT7 alleles. (C) Cortical confocal slices of tobacco abaxial epidermal cells transiently co-expressing mRFP-tagged SYT5 and SYT7 constructs under the control of the UBQ10 promoter and an endoplasmic reticulum (ER) marker (ER-CFP; Nelson et al., 2007). Scale = 5 µm. (D) qPCR showing relative SYT5 and SYT7 transcript levels in sdm26 and sdm34 mutants, normalized to EF1α abundance using the 2-∆∆Ct method. RNA was extracted from 4-week-old rosette leaves of backcrossed sdm26 and sdm34 mutants. Error bars = SD. Groups indicated with the same letters are not significantly different as assessed by ANOVA with Tukey’s post-hoc test. (E, F) MSL10pMSL10-GFP transgenes were introduced into plants heterozygous for the sdm26 (SYT5 S66F) or sdm34 (SYT7 G427R) alleles (the msl10-3G allele had previously been crossed away). Heterozygous T1 plants were identified, and MSL10-GFP stability was compared in T2 siblings that were homozygous for either SYT allele. (E) Deconvolved images of MSL10-GFP signal in leaf epidermal cells of 4-week-old T2 plants. Scale = 10 µm. (F) Immunoblot of MSL10-GFP protein extracted from leaves of 4-week-old T2 siblings. Blots were re-probed with anti-α-tubulin as a loading control. Uncropped images are included as Figure 7—figure supplement 1—source data 1.
-
Figure 7—figure supplement 1—source data 1
Uncropped MSL10-GFP and α-tubulin immunoblots comparing MSL10-GFP expression in plants with and without the SYT5 S66F and SYT7 G427R alleles.
- https://cdn.elifesciences.org/articles/80501/elife-80501-fig7-figsupp1-data1-v2.zip

MSL10 does not interact with SYT5 or SYT7 nor reliably alter their localization.
(A, D) Confocal Z-projections (maximum intensity projection of Z-slices from the top to the middle of cells) of abaxial leaf epidermal cells from 4-week-old plants with the indicated MSL10 alleles. Scale = 15 µm. Quantification of the percentage of the leaf epidermal cell volume taken up by SYT7-GFP (B, C) or SYT5-GFP (E, F) puncta in plants in the msl10-1 or msl10-3G backgrounds compared to WT siblings (A–C) or cousins (D–F). Each data point represents a biological replicate (the mean value of 20–50 epidermal cells from one plant), n = 6–19 plants per genotype from 2 to 4 separately grown flats. Error bars, SD. Means were compared by Student’s t-tests. (G) Mating-based split-ubiquitin assay testing the interaction of MSL10 with SYT5 and SYT7, performed as in Figure 2A. (H) Fluorescence lifetime (τ) of GFP measured using Förster resonance energy transfer-fluorescence lifetime imaging microscopy (FRET-FLIM) when UBQ:MSL10-GFP was transiently expressed in tobacco leaves for 5 days, with or without UBQ:SYT-mRFP . Each data point represents the value from one field of view (three fields of view per plant from three infiltrated plants for a total of n = 9 for each combination). Error bars, SD. Groups indicated by the same letter are not statistically different according to ANOVA with Tukey’s post-hoc test.
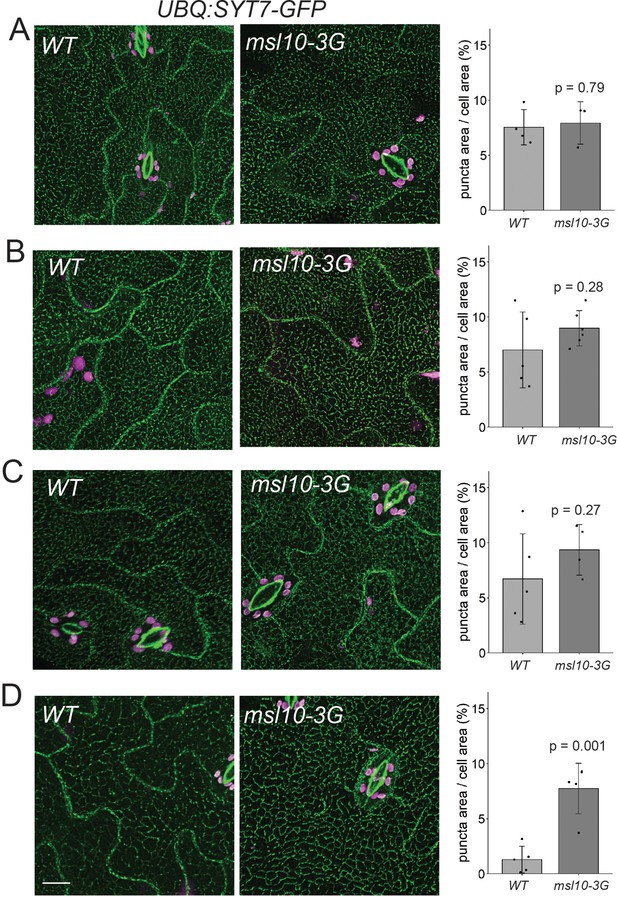
SYT7-GFP localization in leaf epidermal cells varies between experiments.
(A–D) each represent single experiments of separately grown UBQ:SYT7-GFP x msl10-3G F2 siblings. Left: representative deconvolved confocal Z-projections (maximum intensity projection of Z-slices from the top to the middle of cells) of SYT7-GFP fluorescence in 4-week-old abaxial epidermal cells with the indicated genotypes. Right: quantification of the percentage of the leaf epidermal cell volume taken up by SYT7-GFP puncta in plants of each genotype. Each data point represents a biological replicate: the mean value of 10–30 epidermal cells from one plant, 4–6 plants per genotype per experiment. Error bars, SD. Means were compared by Student’s t-tests.
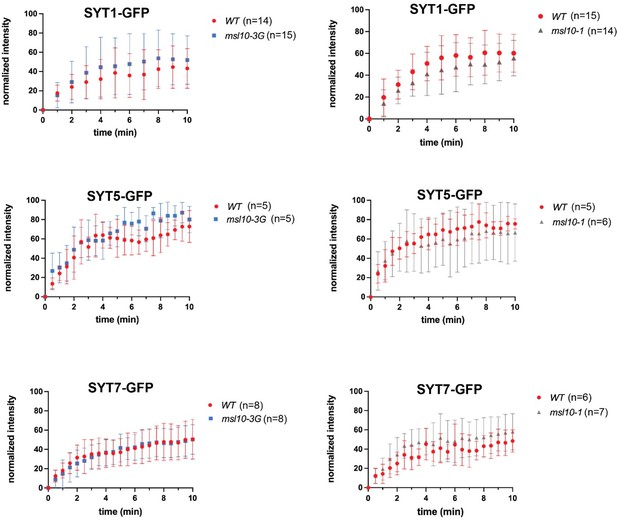
SYT1, SYT5, and SYT7 dynamics are not appreciably altered by MSL10.
Fluorescence recovery after photobleaching (FRAP) curves for SYT1-GFP, SYT5-GFP, and SYT7-GFP in leaf epidermal cells of 4–5-week-old plants with the indicated MSL10 genotypes. The number of individual plants examined is indicated above. The fluorescence intensity of the photobleached ROI was normalized to that of an unbleached, control ROI and by setting the initial fluorescence intensity to 100%. Error bars = SD.
Videos
Time-lapse images of SYT5-mRFP in tobacco abaxial leaf epidermal cells.
Images were taken every 3 s for 2 min, 5 days post-infiltration.
Time-lapse images of SYT5 S66F-mRFP in tobacco abaxial leaf epidermal cells.
Images were taken every 3 s for 2 min, 5 days post-infiltration.
Time-lapse images of SYT7-mRFP in tobacco abaxial leaf epidermal cells.
Images were taken every 3 s for 2 min, 5 days post-infiltration.
Time-lapse images of SYT7 G427R-mRFP in tobacco abaxial leaf epidermal cells.
Images were taken every 3 s for 2 min, 5 days post-infiltration.
Tables
Segregation of MSL10 alleles in crosses to lines overexpressing GFP-labelled endoplasmic reticulum–plasma membrane contact sites (EPCS) proteins.
msl10-1 and msl10-3G plants were crossed to lines expressing GFP-labelled VAP27-1, VAP27-3, SYT1, SYT5, and SYT7 under the control of the UBQ10 promoter. F2 plants (or F3 offspring of heterozygous F2 plants) were selected based on Basta resistance driven by the UBQ:GFP transgenes, and resistant plants were genotyped for the indicated MSL10 alleles. Chi-squared tests were calculated based on a predicted 1:2:1 segregation ratio. Crosses that had significant deviations (Pp<0.05) from expected ratios are in bold.
| # Basta resistant offspring with indicated genotypes | ||||||
|---|---|---|---|---|---|---|
| Parental genotype | MSL10/MSL10 | MSL10/msl10-3G | msl10-3G/msl10-3G | X2 | P | |
| UBQ:VAP27-1-GFP/-; | MSL10/msl10-3G | 6/25 (24%) | 16/25 (64%) | 3/25 (12%) | 2.68 | 0.26 |
| UBQ:VAP27-3-GFP/-; | MSL10/msl10-3G | 12/33 (36%) | 21/33 (64%) | 0/33 (0%) | 11.18 | 0.004 |
| UBQ:SYT1-GFP/-; | MSL10/msl10-3G | 6/21 (29%) | 12/21 (57%) | 3/21 (14%) | 1.29 | 0.53 |
| UBQ:SYT5-GFP/-; | MSL10/msl10-3G | 5/21 (24%) | 7/21 (33%) | 9/21 (43%) | 3.86 | 0.15 |
| UBQ:SYT7-GFP/-; | MSL10/msl10-3G | 9/40 (23%) | 23/40 (57%) | 8/40 (20%) | 0.95 | 0.62 |
| MSL10/MSL10 | MSL10/msl10-1 | msl10-1/msl10-1 | ||||
| UBQ:VAP27-1-GFP/-; | MSL10/msl10-1 | 6/28 (21%) | 17/28 (61%) | 5/28 (18%) | 1.36 | 0.51 |
| UBQ:VAP27-3-GFP/-; | MSL10/msl10-1 | 7/36 (19%) | 29/36 (81%) | 0/36 (0%) | 16.17 | 0.0003 |
| UBQ:SYT1-GFP/-; | MSL10/msl10-1 | 24/74 (33%) | 46/74 (62%) | 4/74 (5%) | 15.19 | 0.0005 |
| UBQ:SYT5-GFP/-; | MSL10/msl10-1 | 7/23 (30%) | 8/23 (35%) | 8/23 (35%) | 2.22 | 0.33 |
| UBQ:SYT7-GFP/-; | MSL10/msl10-1 | 16/42 (38%) | 17/42 (41%) | 9/42 (21%) | 3.86 | 0.15 |
| Expected ratios | 25% | 50% | 25% | |||
Primers used in subcloning, genotyping, and sequencing.
| # | Name | Sequence (5' → 3') | Purpose |
|---|---|---|---|
| 2229 | LBb1.3 | ATTTTGCCGATTTCGGAAC | Genotyping SALK T-DNA insertion lines |
| 3623 | msl10 salk F | GTTGGTTTCTGGGTTTAAGCC | msl10-1 genotyping |
| 3624 | msl10 salk R | TACTTGGAGTAACCGGTGCTG | msl10-1 genotyping |
| 702 | MSL10 exon2 For | GCAACGACTAAGGTTTTGCTG | msl10-3G genotyping (for CAPS with Taq1 digestion) |
| 663 | MSL10 exon4 Rev | GTTCTTCTTTGTGAGATTAATGTCTTGAGG | msl10-3G genotyping (for CAPS with Taq1 digestion), sequencing of MSL10 genomic DNA |
| 1214 | LB1.SAIL | GCTTTTCAGAAATGGATAAATAGCCTTGCTTCC | Genotyping SAIL T-DNA insertion lines |
| 4127 | syt1 genotyping F | GAATTGTCCATGTGAAAGTTGTG | syt1 genotyping |
| 4128 | syt5 genotyping F | CTGTCAGCGTTTCTCTTAGAG | syt5 genotyping |
| 4129 | syt5 genotyping R | GAAGAACGTCAACAGTTCAA | syt5 genotyping |
| 4130 | syt7 genotyping F | GAGAAAGCACTAGATAGTTTGACG | syt7 genotyping |
| 4131 | syt7 genotyping R | CTGCTGTTTTGCACCATC | syt7 genotyping |
| 4055 | VAP27-1 For | CACCATGAGTAACATCGATCTGATTG | Amplification of VAP27-1 ORF for pENTR/D-TOPO cloning |
| 3993 | VAP27-1 Rev | TGTCCTCTTCATAATGTATCCC | Amplification of VAP27-1 ORF for pENTR/D-TOPO cloning |
| 3988 | VAP27-3 For | CACCATGAGTAACGAGCTTCTCAC | Amplification of VAP27-3 ORF for pENTR/D-TOPO cloning |
| 4053 | VAP27-3 Rev | TTATGTCCTCTTCATAATGTATCC | Amplification of VAP27-3 ORF for pENTR/D-TOPO cloning |
| 3990 | SYT1 For | CACCATGGGCTTTTTCAGTACGATAC | Amplification of SYT1 ORF for pENTR/D-TOPO cloning |
| 3991 | SYT1 Rev | AGAGGCAGTTCGCCACTC | Amplification of SYT1 ORF for pENTR/D-TOPO cloning / syt1 genotyping |
| 4038 | ACT8 For | CACCATGGCCGATGCTGATGAC | Amplification of ACT8 ORF for pENTR/D-TOPO cloning |
| 4039 | ACT8 Rev | TTAGAAGCATTTTCTGTGGACAATGA | Amplification of ACT8 ORF for pENTR/D-TOPO cloning |
| 4024 | DL1 For | CACCATGGAAAATCTGATCTCTCTGGT | Amplification of DL1 ORF for pENTR/D-TOPO cloning |
| 4025 | DL1 Rev | CTTGGACCAAGCAACAGC | Amplification of DL1 ORF for pENTR/D-TOPO cloning |
| 4026 | RAB1c For | CACCATGAATCCTGAATATGACTATTTGTT | Amplification of RAB1c ORF for pENTR/D-TOPO cloning |
| 4027 | RAB1c Rev | TTAAGAGGAGCAGCAGCC | Amplification of RAB1c ORF for pENTR/D-TOPO cloning |
| 4020 | aCOP1 For | CACCATGTTGACAAAGTTCGAAACC | Amplification of COPA1 ORF for pENTR/D-TOPO cloning |
| 4052 | aCOP1 Rev | CCGGACTTGAGATGGAGAGCATA | Amplification of COPA1 ORF for pENTR/D-TOPO cloning |
| 4030 | LOS1 For | CACCATGGTGAAGTTTACAGCTG | Amplification of LOS1 ORF for pENTR/D-TOPO cloning |
| 4031 | LOS1 Rev | TTAAAGCTTGTCTTCGAAC | Amplification of LOS1 ORF for pENTR/D-TOPO cloning |
| 4036 | MTO3 For | CACCATGGAATCTTTTTTGTTCAC | Amplification of MTO3 ORF for pENTR/D-TOPO cloning |
| 4037 | MTO3 Rev | AGCTTGGACCTTGTTAGAC | Amplification of MTO3 ORF for pENTR/D-TOPO cloning |
| 3986 | AT3G44330 For | CACCATGGCGGAAGAGAAGAAAT | Amplification of M28 peptidase ORF for pENTR/D-TOPO cloning |
| 3987 | AT3G44330 Rev | TCCCATTTTCACTTTCCG | Amplification of M28 peptidase ORF for pENTR/D-TOPO cloning |
| 4032 | RPT1a For | CACCATGGTGAGAGATATTGAAGAT | Amplification of RPT1a ORF for pENTR/D-TOPO cloning |
| 4033 | RPT1a Rev | ATTGTAGACCATATACTTGGG | Amplification of RPT1a ORF for pENTR/D-TOPO cloning |
| 4028 | CAT2 For | CACCATGGATCCTTACAAGTATCGTC | Amplification of CAT2 ORF for pENTR/D-TOPO cloning |
| 4029 | CAT2 Rev | TTAGATGCTTGGTCTCACG | Amplification of CAT2 ORF for pENTR/D-TOPO cloning |
| 3994 | AT3G62360 For | CACCATGGCGGCCAGTAGGAAG | Amplification of AT3G44330 ORF for pENTR/D-TOPO cloning |
| 3995 | AT3G62360 Rev | GAACGTCTTCTTTCTAGCAACAGC | Amplification of AT3G44330 ORF for pENTR/D-TOPO cloning |
| 4022 | RAN1 For | CACCATGGCTCTACCTAACCAG | Amplification of RAN1 ORF for pENTR/D-TOPO cloning |
| 4023 | RAN1 Rev | CTCAAAGATATCATCATCGTC | Amplification of RAN1 ORF for pENTR/D-TOPO cloning |
| 3781 | MSL10g upstream seq For | CCCACAGTGTTCTTCTATAATC | Amplification of MSL10 genomic DNA |
| 3782 | MSL10g downstream seq Rev | CAGTATCACAACGTTTGGTA | Amplification of MSL10 genomic DNA |
| 699 | MSL10 exon1 For | CAGCACCGGTTACTCCAAGT | Sequencing of MSL10 genomic DNA |
| 701 | MSL10 exon1 For2 | ACACATTGGACGAAACAGCA | Sequencing of MSL10 genomic DNA |
| 1611 | MSL10 exon1 Rev | GTTATTGACGTTGAAATTCGCTGCAAGG | Sequencing of MSL10 genomic DNA |
| 2227 | MSL10 exon3 Rev | CGGACTTCTGAAGTAAGCGCTTATCGGTTTCGTGG | Sequencing of MSL10 genomic DNA |
| 3789 | MSL10 intron2 Rev | CCATAATTTATCTTTAAAGAATAAAAGCATG | Sequencing of MSL10 genomic DNA |
| 4145 | SYT5 S66F For | CCTGGGTTGTCTTCTTCGAGCGTCAGAAGTTG | Introducing S66F mutation into SYT5 by site-directed mutagenesis |
| 4146 | SYT5 S66F Rev | CAACTTCTGACGCTCGAAGAAGACAACCCAGG | Introducing S66F mutation into SYT5 by site-directed mutagenesis |
| 4147 | SYT7 G427R For | CAATGGATGCAGTCAGGATGGTGGGAAGTGG | Introducing G427R mutation into SYT7 by site-directed mutagenesis |
| 4148 | SYT7 G427R Rev | CCACTTCCCACCATCCTGACTGCATCCATTG | Introducing G427R mutation into SYT7 by site-directed mutagenesis |
| 4155 | SYT5 For | CACCATGGGTTTCATAGTCGGC | Amplifying SYT5 for S66F CAPs genotyping/ SYT5 Gateway cloning |
| 4156 | SYT5 internal rev | ACATAAGGCCAGATCTTTGTC | Amplifying SYT5 for S66F CAPs genotyping/ SYT5 Gateway cloning |
| 4231 | SYT7 dCAPs For | GTAGCACAATGGATGCACTC | Amplifying SYT7 for G427R dCAPs genotyping |
| 4232 | SYT7 internal Rev | ATCCACTACCGACCGCTC | Amplifying SYT7 for G427R dCAPs genotyping |
| 4157 | SYT5 Rev | GGAATCACGATAAATTGATTGA | Amplification of SYT5 for pENTR/D-TOPO cloning |
| 4158 | SYT7 For | CACCATGGGTTTGATTTCTGGG | Amplification of SYT7 for pENTR/D-TOPO cloning |
| 4159 | SYT7 Rev | CTGCTGTTTTGCACCATC | Amplification of SYT7 for pENTR/D-TOPO cloning |
| 1758 | attB1-F | ACAAGTTTGTACAAAAAAGCAGGCTCTCCAACCACCATG | Amplifying genes for split-ubiquitin cloning in yeast |
| 1759 | attB2-R | TCCGCCACCACCAACCACTTTGTACAAGAAAGCTGGGTA | Amplifying genes for split-ubiquitin cloning in yeast |
| 3196 | EF1α qRT For | ACAGGCGTTCTGGTAAGGAG | Amplifying EF1α transcripts for qPCR |
| 3197 | EF1α qRT Rev | CCTTCTTCACTGCAGCCTTG | Amplifying EF1α transcripts for qPCR |
| 4442 | SYT5 qRT For | AGAGGTGAAGCTTGTGCAAG | Amplifying SYT5 transcripts for qPCR |
| 4443 | SYT5 qRT Rev | TGTTGAGTTGACGCGTCTTC | Amplifying SYT5 transcripts for qPCR |
| 4444 | SYT7 qRT For | GCCTTGGACTTGTGAAACTTCC | Amplifying SYT7 transcripts for qPCR |
| 4445 | SYT7 qRT Rev | TCTTCCAACGCAGCCATTTG | Amplifying SYT7 transcripts for qPCR |