An international observational study to assess the impact of the Omicron variant emergence on the clinical epidemiology of COVID-19 in hospitalised patients
Figures
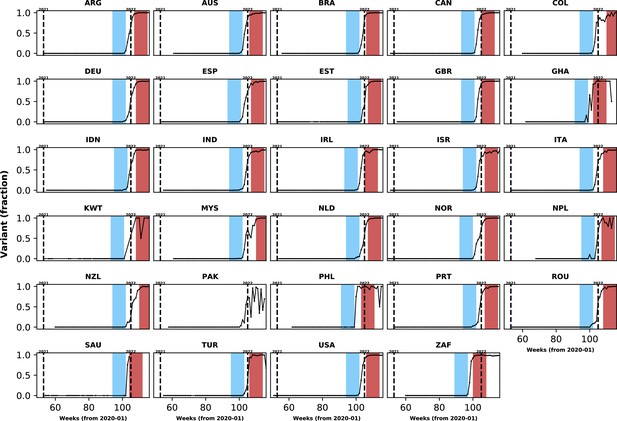
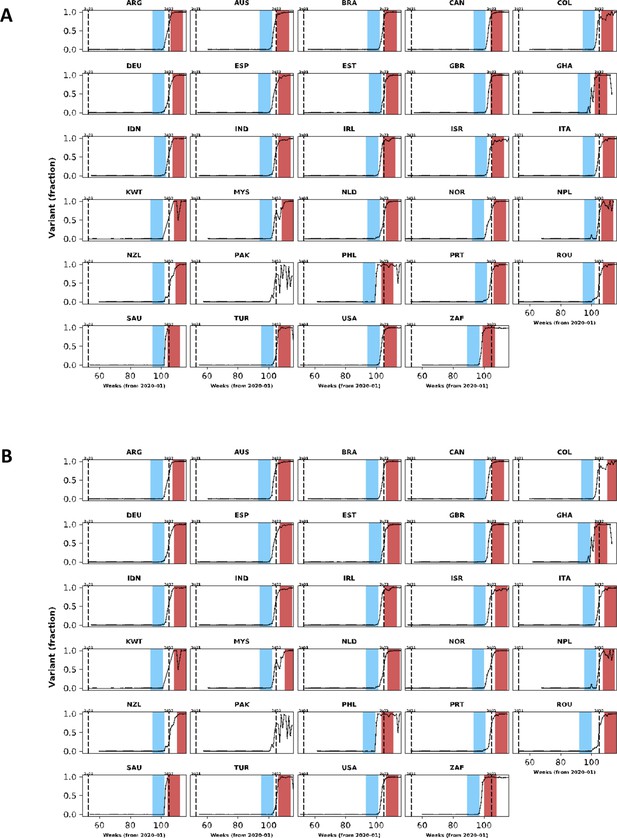
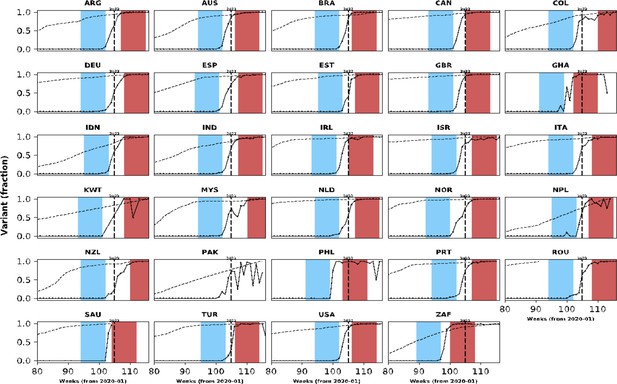
Population-level relative frequency of Omicron variant infections by country and time.
Here, data aggregated by epidemiological week and country were used to calculate the proportions of infections caused by the Omicron variant. For analyses reported in the Results section, two epidemiological periods were defined: the first corresponds to the two months before the Omicron variant reaches a threshold frequency of 10% (blue area in each panel; the pre-Omicron period); the second period corresponds to the two months after Omicron variant frequency reaches 90% (red area in each panel; the Omicron period). Sensitivity analyses, using other relative frequencies for defining periods, are presented in the Appendix 1. Each panel presents data for a country (ISO3 code as title) contributing clinical data for this analysis; y-axes represent proportions in each epidemiological week (x-axes). Data for Laos are not shown as, at the time of the analysis, samples were not included in the database that informed population-level frequency of Omicron variant during the study period. In Pakistan, due to fluctuations in Omicron variant frequency in the dataset, study periods were not defined. More information on the spread of the Omicron variant in Laos and analysis of the clinical data from Pakistan are presented in the Appendix 1.
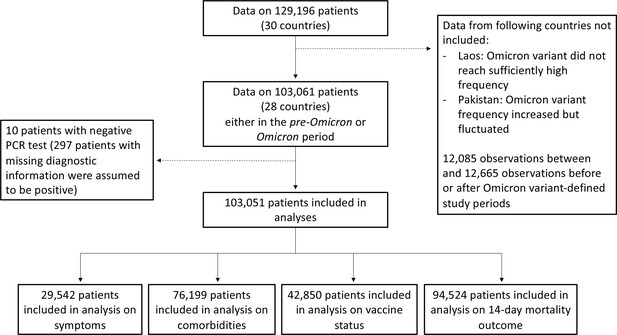
Study flowchart.
In this figure, we present the numbers of observations included in analyses in the different subsections of the Results section.
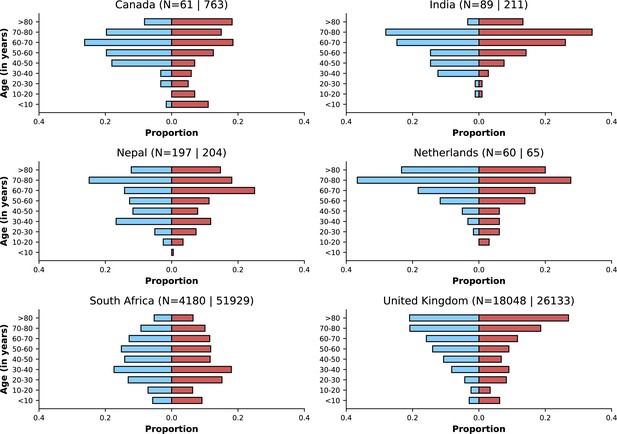
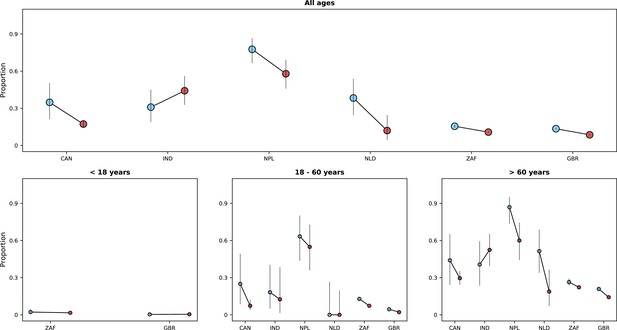
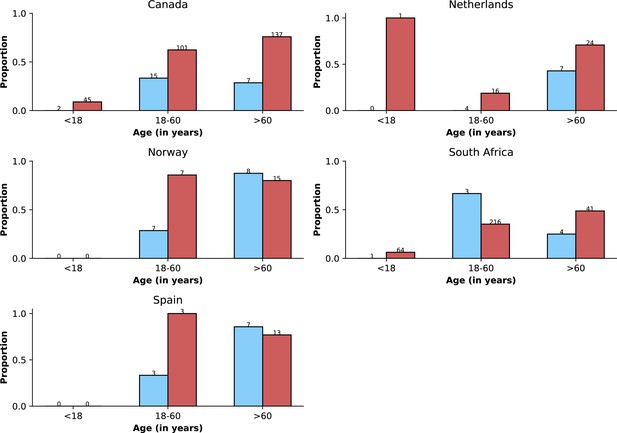
Age distributions by study period and country.
Age distributions (x-axes show proportions; y-axes, age groups) when Omicron variant relative frequency was below 10% (blue bars) and when the frequency was 90% or higher (red bars). Data from different countries are shown in different panels; only countries with 50 or more records in each period are presented. Numbers of observations with age information are shown for each study period next to country names.
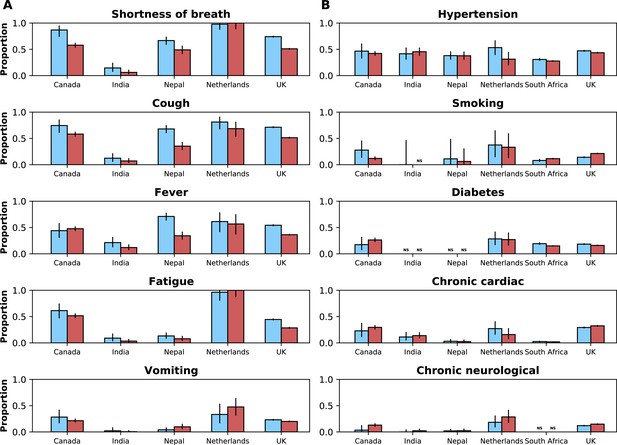
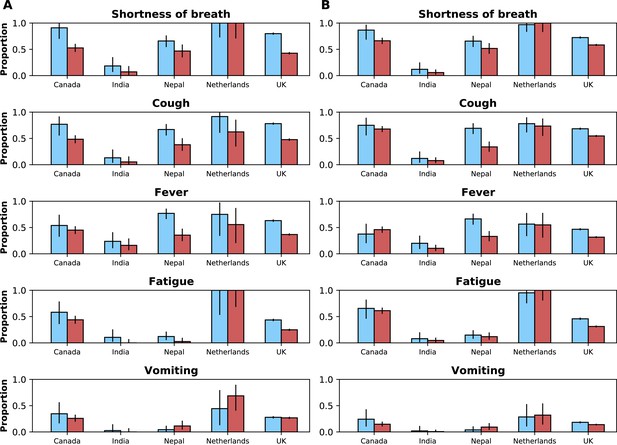
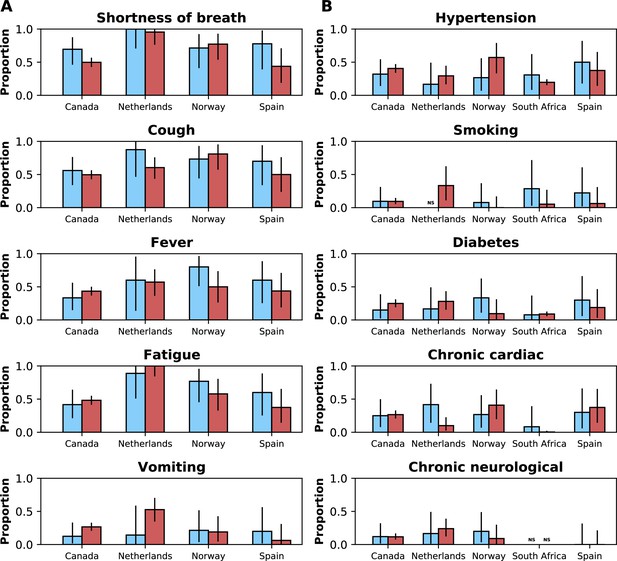
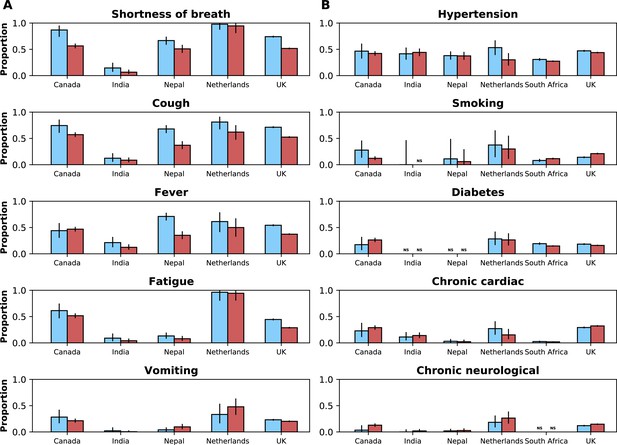
Frequencies of symptoms and comorbidities by study period and country.
Frequencies of the five most common symptoms (A) and comorbidities (B) during the pre-Omicron (blue bars) and Omicron (red bars) periods. 95% confidence intervals are shown. Note that South Africa is included in panel B but not panel A. For panel (A), only data from the pre-Omicron period were used to identify the most frequent symptoms; for panel (B), as data on comorbidities were available in the two countries contributing most records, the United Kingdom and South Africa, and since their relative contributions to the study population changed in the two study periods, the dataset including both the pre-Omicron and Omicron periods was used to identify most common comorbidities. Only countries with at least 50 observations during each study period are included. For each symptom or comorbidity, whenever fewer than five observations without missing data were available, bars were not shown and the text ‘NS’ (not shown) was included.
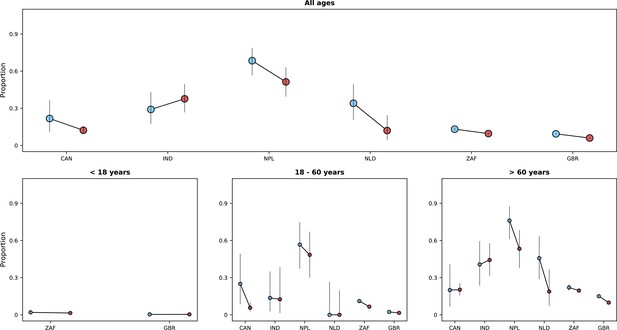
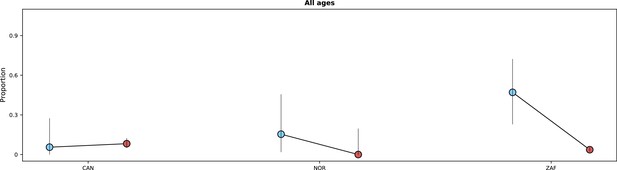
Risk of death (y-axes) in the first 14 days after hospital admission or disease onset, whichever occurred latest, during the pre-Omicron and Omicron periods.
In each panel, the x-axis shows countries (ISO3 codes are presented), with different periods represented by circles with different colours (blue circles for the pre-Omicron period; red circles, for the Omicron period). 95% confidence intervals are also presented. The top panel shows data for individuals of all ages; the bottom panels, data for patients aged less than 18 years, between 18 and 60 years, and older than 60 years. Only countries with at least 50 observations in both study periods are included in the figure; for panels presenting age-specific estimates (bottom row), a further requirement for inclusion was outcome data for at least 10 patients in the corresponding age range in both periods.
In this figure, population-level variant data are presented for countries with clinical data included in our analysis.
The same structure of Figure 1 was used but different cut-off frequencies for Omicron variant were applied: in (A), the lower and upper threshold frequencies were 10% and 80%; in (B), these frequencies were 5% and 90%.
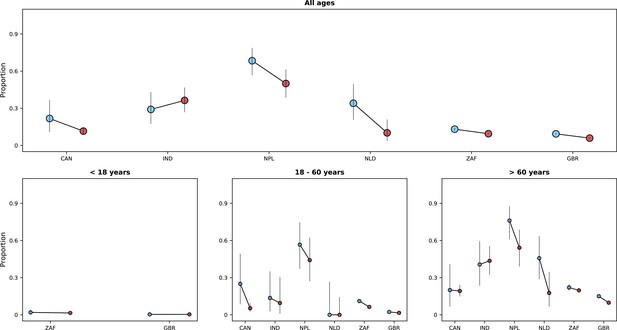
Frequencies of the five most common symptoms during the period before (blue bars) and after (red bars) Omicron variant frequency reached 10% and 90%, respectively.
95% confidence intervals are also shown. In (A), data from individuals aged between 18 and 60 years are shown; and (B) shows the same information for individuals older than 60 years. Data from children are not presented.
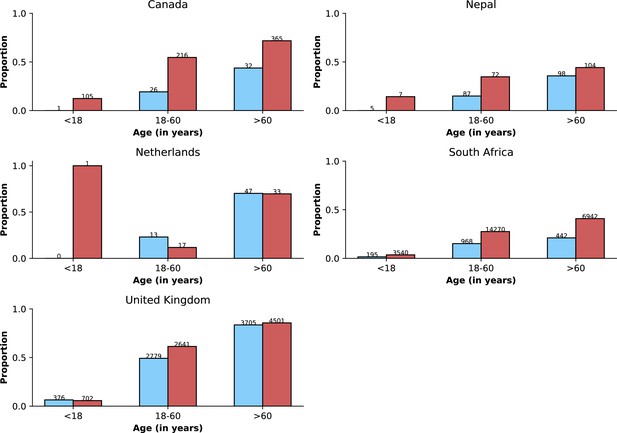
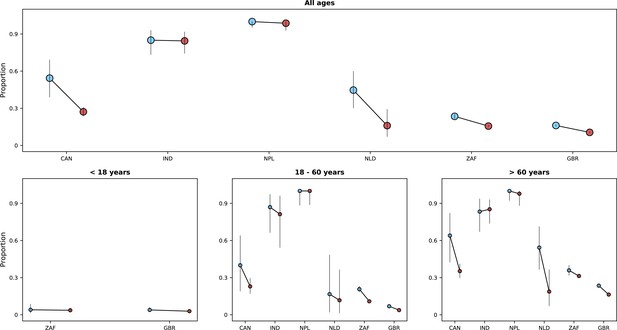
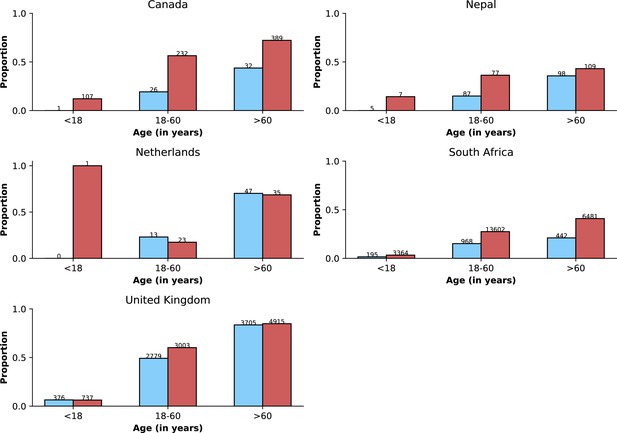
Frequency of previous vaccination by study period, age category and country.
Only data from countries with at least 50 observations with information on previous vaccination during both study periods defined by Omicron variant frequency are shown. In each panel, the x-axis shows different age categories, with blue bars corresponding to the pre-Omicron period and red bars, to the period after Omicron variant frequency, relative to other variants, reaches 90%. Above each bar, the total number of records included in the calculation of the proportions (y-axes) are presented.
Population-level vaccination coverage.
Data from different countries are presented in different panels; x-axes show epidemiological weeks since the first epidemiological week of 2020. As in Figure 1, continuous black lines represent frequency of Omicron variant relative to the other variants. In addition to information on Omicron variant frequency, each panel also shows data on vaccination: the dashed line shows the proportion of population vaccinated with at least one dose relative to the maximum number vaccinated in each country at the time of the analysis (March 2022). Data used to generate this figure were downloaded from https://ourworldindata.org/.
Risk of death in the first 28 days after hospital admission or disease onset, whichever occurred latest, during pre-Omicron and Omicron periods.
In each panel, the x-axis shows countries, with different periods represented by circles with different colours (blue circles for the pre-Omicron period; red circles, for period after Omicron variant frequency reaches 90%). 95% confidence intervals are presented. The top panel shows data for individuals of all ages; the bottom panels, data for patients aged less than 18 years, between 18 and 60 years, and older than 60 years. Only countries with at least 50 observations in both study periods are included in the figure; for panels presenting age-specific estimates (bottom row), a further requirement for inclusion was outcome data for at least 10 patients in the corresponding age range in both periods.
Risk of death or invasive mechanical ventilation by study period.
In each panel, the x-axis shows countries, with different periods represented by circles with different colours (blue circles for the pre-Omicron period; red circles, for the Omicron period). 95% confidence intervals are presented. The top panel shows data for individuals of all ages; the bottom panels, data for patients aged less than 18 years, between 18 and 60 years, and older than 60 years. Only countries with at least 50 observations in both study periods are included in the figure; for panels presenting age-specific estimates (bottom row), a further requirement for inclusion was outcome data for at least 10 patients in the corresponding age range in both periods. Different from Figure 5 and Appendix 1—figure 5, time since hospital admission or onset of symptoms was not used since for most patients who required invasive mechanical ventilation the start date of the therapeutic approach was not available. Only patients with information on invasive mechanical ventilation use and who were either discharged or died were included.
This figure shows similar information to that presented in Figure 3.
The legend of that figure applies to this figure, except that instead of referring to time periods, the figure shows data for Delta and Omicron variants. Only countries with at least 10 observations for Delta and Omicron variants are included.
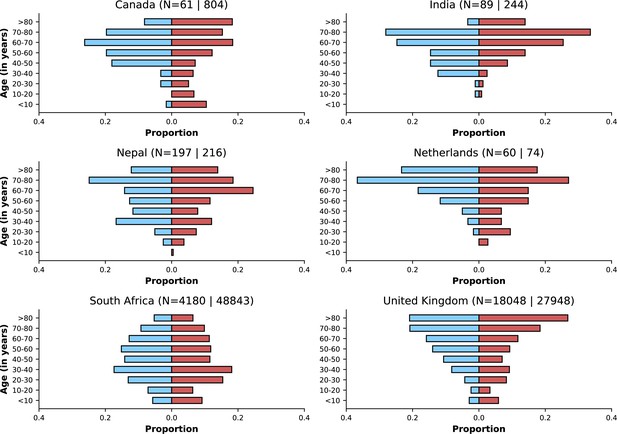
This figure shows similar information to that presented in Figure 4.
The legend of that figure applies to this figure, except that instead of referring to time periods, the figure shows data for Delta and Omicron variants. Only countries with at least 10 observations for Delta and Omicron variants are included.
This figure shows similar information to that presented in Appendix 1—figure 3.
The legend of that figure applies to this figure, except that instead of referring to time periods, the figure shows data for Delta and Omicron variants. Only countries with at least 10 observations for Delta and Omicron variants are included; note that, different from Appendix 1—figure 3, the criterion did not consider missingness of vaccination data.
This figure shows similar information to that presented in Figure 5.
The legend of that figure applies to this figure, except that instead of referring to time periods, the figure shows data for Delta and Omicron variants. Only countries with at least 10 observations for Delta and Omicron variants are included. Age-stratified panels are not shown due to the limited number of observations with individual-level variant data.
This figure shows similar information to that presented in Figure 3.
The legend of that figure applies to this figure. Here, the upper threshold frequency used to define Omicron variant dominance was 80% rather than 90%.
This figure shows similar information to that presented in Figure 4.
The legend of that figure applies to this figure. Here, the upper threshold frequency used to define Omicron variant dominance was 80% rather than 90%.
This figure shows similar information to that presented in Appendix 1—figure 3.
The legend of that figure applies to this figure. Here, the upper threshold frequency used to define Omicron variant dominance was 80% rather than 90%.
This figure shows similar information to that presented in Figure 5.
The legend of that figure applies to this figure. Here, the upper threshold frequency used to define Omicron variant dominance was 80% rather than 90%.
Tables
Vaccination status by country and study period.
Data for period-country combinations with less than 10 observations are not presented. Data on vaccination status were not available for patients from Saudi Arabia.
| pre-Omicron period | Omicron period | |||
|---|---|---|---|---|
| Country | % Vaccinated | Total N | % Vaccinated | Total N |
| Brazil | 84.6 | 13 | 87.9 | 33 |
| Canada | 32.2 | 59 | 57.3 | 686 |
| Colombia | 42.1 | 19 | - | <10 |
| Estonia | - | <10 | ||
| Germany | - | <10 | - | <10 |
| India | 34.8 | 23 | 84.8 | 33 |
| Malaysia | 79.3 | 29 | 80.0 | 10 |
| Nepal | 25.3 | 190 | 39.3 | 183 |
| Netherlands | 60.0 | 60 | 51.0 | 51 |
| New Zealand | 5.9 | 34 | - | <10 |
| Norway | - | <10 | 82.2 | 45 |
| Philippines | 78.6 | 14 | - | <10 |
| Portugal | - | <10 | - | <10 |
| Romania | - | <10 | 78.6 | 98 |
| South Africa | 15.1 | 1605 | 27.9 | 24752 |
| Spain | 45.0 | 20 | 70.9 | 55 |
| United Kingdom | 65.4 | 6865 | 70.3 | 7846 |
| United States of America | - | <10 | - | <10 |
| Argentina | - | <10 | ||
| Australia | - | <10 | ||
| Indonesia | - | <10 | ||
| Israel | 54.5 | 11 | ||
| Kuwait | 66.7 | 18 | ||
| Turkey | 74.1 | 27 | ||
Odds ratio for the association between study period and mortality outcome.
Results of multivariate logistic models, with random intercepts for countries, on 14-day fatality risk are presented. Different models were fit that included different variables. Model III adjusts for all variables in the table, however due to missing data in the vaccination and comorbidity variables, less than a third of the study population was included in the estimation of that model; models I and II were thus fit that did not adjust for these variables and included more individuals. In model IV, a category for missing data was created for the variable on previous vaccination; individuals in that category had an odds ratio of 0.74 (0.69–0.80; reference group in this comparison is the non-vaccinated group). Note that similar results were obtained when finer categorisation of the age variable, 10-year intervals, was used. As previous SARS-CoV-2 infection has been shown to reduce severity of COVID-19 (Altarawneh et al., 2022), a multivariable model that also adjusted for this variable was fit; in that model, the odds ratio for the association between study period and fatality risk was 0.70 (0.61–0.80). As in other epidemiological studies, estimates for covariates other than the primary exposure (study period) should be carefully interpreted (Westreich and Greenland, 2013).
| Model | I | II | III | IV |
|---|---|---|---|---|
| Number of observations | 94,077 | 39,950 | 26,728 | 56,329 |
| Odds ratio (95% CI) | Odds ratio (95% CI) | Odds ratio (95% CI) | Odds ratio (95% CI) | |
| Variables | ||||
| Omicron period* | 0.65 (0.62–0.69) | 0.67 (0.61–0.75) | 0.68 (0.60–0.77) | 0.64 (0.59–0.69) |
| Sex (male) | 1.32 (1.26–1.38) | 1.33 (1.23–1.43) | 1.36 (1.24–1.49) | 1.33 (1.25–1.42) |
| Age | ||||
| Older than 60 years | Reference | Reference | Reference | Reference |
| Aged between 18 and 60 years | 0.26 (0.25–0.27) | 0.24 (0.22–0.26) | 0.27 (0.25–0.30) | 0.30 (0.27–0.32) |
| Younger than 18 years | 0.06 (0.05–0.07) | 0.06 (0.05–0.07) | 0.07 (0.05–0.09) | 0.06 (0.05–0.08) |
| Previous vaccination | - | 0.60 (0.55–0.65) | 0.53 (0.48–0.59) | 0.59 (0.54–0.65) |
| Comorbidities | ||||
| Hypertension | - | - | 1.29 (1.16–1.42) | 1.26 (1.17–1.35) |
| Diabetes | - | - | 1.22 (1.09–1.38) | 1.22 (1.12–1.32) |
| Chronic cardiac disease | - | - | 1.50 (1.31–1.71) | 1.51 (1.39–1.65) |
-
*
Odds ratio in univariate analysis 0.65 (0.61–0.69) (N=94,524).
Numbers of records contributed by partner institutions in different countries between 01/10/2021 and 28/02/2022.
| Country | Number of records |
|---|---|
| South Africa | 69766 |
| United Kingdom | 55049 |
| Pakistan | 929 |
| Canada | 919 |
| Nepal | 504 |
| Laos | 456 |
| India | 409 |
| Romania | 166 |
| Saudi Arabia | 151 |
| Spain | 151 |
| Netherlands | 134 |
| Malaysia | 90 |
| Norway | 67 |
| Turkey | 57 |
| Brazil | 54 |
| Colombia | 52 |
| New Zealand | 46 |
| Kuwait | 35 |
| United States | 32 |
| Philippines | 26 |
| Ghana | 21 |
| Ireland | 20 |
| Israel | 17 |
| Italy | 12 |
| Estonia | 7 |
| Australia | 7 |
| Indonesia | 6 |
| Portugal | 5 |
| Germany | 4 |
| Argentina | 4 |
Missing data on symptoms.
Note that this information was not systematically recorded in South Africa, and for this reason data from that country are not included in this table.
| Symptoms | Yes | No | Missing data |
|---|---|---|---|
| Any cough | 20431 | 13726 | 25273 |
| Fever | 16045 | 19465 | 23920 |
| Headache | 3896 | 28398 | 27136 |
| Confusion | 5960 | 28548 | 24922 |
| Seizures | 570 | 33424 | 25436 |
| Sore throat | 2394 | 29353 | 27683 |
| Runny nose | 1639 | 30279 | 27512 |
| Vomiting | 6956 | 27734 | 24740 |
| Wheezing | 2042 | 31191 | 26197 |
| Diarrhoea | 4418 | 29989 | 25023 |
| Chest pain | 5488 | 28732 | 25210 |
| Conjunctivitis | 106 | 32606 | 26718 |
| Myalgia | 3686 | 28195 | 27549 |
| Rash | 476 | 32877 | 26077 |
| Fatigue | 11339 | 22150 | 25941 |
| Ageusia | 1682 | 28341 | 29407 |
| Inability to walk | 252 | 3797 | 55381 |
| Anosmia | 1393 | 29040 | 28997 |
| Shortness of breath | 20490 | 14030 | 24910 |
| Lymphadenopathy | 145 | 32795 | 26490 |
Missing data on comorbidities.
In this table, data from all countries are included.
| Comorbidities | Yes | No | Missing data |
|---|---|---|---|
| Liver disease | 1786 | 40992 | 86418 |
| Diabetes | 12956 | 68743 | 47497 |
| Chronic cardiac disease | 13546 | 73423 | 42227 |
| Hypertension | 32052 | 57401 | 39743 |
| Current smoking | 5090 | 26674 | 97432 |
| COPD | 9304 | 77794 | 42098 |
| Active TB | 1579 | 45731 | 81886 |
| Asthma | 8720 | 79175 | 41301 |
| Chronic kidney disease | 8441 | 78453 | 42302 |
| Malignant neoplasm | 5062 | 81465 | 42669 |
| Dementia | 4646 | 38530 | 86020 |
| HIV | 5925 | 79121 | 44150 |
| Chronic neurological disorder | 5615 | 37740 | 85841 |
| Obesity | 5723 | 45367 | 78106 |
Potential limitations of population-level variant data used to determine time periods when Omicron variant was dominant.
| Potential limitation | Likely impact on analyses |
|---|---|
| Population-level data come from a range of sources in each country, and for most samples it is not possible to determine whether patient was hospitalised or was a community (mild) case | If different variants are associated with different severities upon infection and if a large fraction of samples used in the estimation of population-level frequency of variants are from community cases, then it is possible that this frequency does not fully represent the frequency in the hospitalised population. In particular, if Omicron variant infection is linked to lower risk of hospitalisation, as previous studies suggest, it is possible that even during periods when community-level frequency of Omicron variant was high, the frequency of Omicron variant in the hospitalised population might have been relatively low. |
| Use of country-level data, rather than data on variant frequency in the catchment areas of clinical centres contributing data | If Omicron variant spreads asynchronously in a country, with some regions reaching high relative frequency faster than others, it is possible that country-level data, rather than data at a finer geographical level, might not reflect Omicron variant frequency in the population from which patients were recruited. |
| Delay between infection, onset of symptoms and hospitalisation | Depending on the data source used to define population-level frequency of variants, if clinical samples were obtained early during the infection, hospitalised cases might only have the same variant composition after a time lag, corresponding to average time from infection, or onset of symptoms, to hospital admission. |
Numbers of records in the pre-Omicron and Omicron periods by country.
| Omicron emergence | |||
|---|---|---|---|
| Country | Before 10% | After 90% | Total |
| South Africa | 4180 | 51929 | 56109 |
| United Kingdom | 18124 | 26479 | 44603 |
| Canada | 61 | 763 | 824 |
| Nepal | 197 | 204 | 401 |
| India | 89 | 212 | 301 |
| Netherlands | 60 | 65 | 125 |
| Saudi Arabia | 2 | 121 | 123 |
| Romania | 1 | 100 | 101 |
| Spain | 21 | 56 | 77 |
| Malaysia | 42 | 11 | 53 |
| Norway | 5 | 45 | 50 |
| Brazil | 15 | 33 | 48 |
| New Zealand | 34 | 6 | 40 |
| Colombia | 26 | 5 | 31 |
| Turkey | 0 | 27 | 27 |
| Philippines | 16 | 5 | 21 |
| United States of America | 14 | 7 | 21 |
| Kuwait | 0 | 19 | 19 |
| Ghana | 4 | 15 | 19 |
| Ireland | 14 | 3 | 17 |
| Israel | 0 | 14 | 14 |
| Australia | 0 | 6 | 6 |
| Portugal | 3 | 2 | 5 |
| Indonesia | 1 | 4 | 5 |
| Germany | 2 | 2 | 4 |
| Italy | 3 | 0 | 3 |
| Argentina | 0 | 3 | 3 |
| Estonia | 1 | 0 | 1 |
Medians (interquartile ranges [Q1 - Q3]) of age by study period and country.
Only countries with 10 or more observations in both study periods are shown.
| Before 10% | After 90% | |||||
|---|---|---|---|---|---|---|
| Country | Median | Q1 | Q3 | Median | Q1 | Q3 |
| Brazil | 59 | 50 | 70 | 55 | 48 | 70 |
| Canada | 63 | 50 | 71 | 62 | 35 | 76 |
| Spain | 68 | 63 | 75 | 76 | 59 | 84 |
| United Kingdom | 66 | 48 | 78 | 67 | 38 | 81 |
| India | 63 | 47 | 72 | 70 | 60 | 76 |
| Malaysia | 63 | 52 | 68 | 59 | 55 | 63 |
| Netherlands | 74 | 64 | 80 | 70 | 55 | 77 |
| Nepal | 63 | 42 | 77 | 64 | 42 | 75 |
| South Africa | 45 | 30 | 62 | 41 | 27 | 63 |
Numbers of hospitalised patients admitted due to COVID-19.
For country-time period combinations with less than 10 observations, numbers are not presented.
| Before 10% | After 90% | |||||
|---|---|---|---|---|---|---|
| Country | COVID-19 as reason (N) | COVID-19 as reason (%) | Total | COVID-19 as reason (N) | COVID-19 as reason (%) | Total |
| Australia | - | - | <10 | |||
| Argentina | - | - | <10 | |||
| Brazil | 14 | 100 | 14 | 32 | 96.7 | 33 |
| Canada | 12 | 52.2 | 23 | 514 | 67.5 | 761 |
| Colombia | 2 | 7.7 | 26 | - | - | <10 |
| Germany | - | - | <10 | - | - | <10 |
| Ghana | - | - | <10 | |||
| India | 0 | 0 | 12 | 2 | 8.3 | 24 |
| Indonesia | - | - | <10 | |||
| Israel | 8 | 66.7 | 12 | |||
| Kuwait | 0 | 0 | 18 | |||
| Malaysia | - | - | <10 | - | - | <10 |
| Nepal | - | - | <10 | 0 | 0 | 15 |
| Netherlands | 49 | 81.7 | 60 | 39 | 61.9 | 63 |
| New Zealand | 30 | 90.9 | 33 | - | - | <10 |
| Norway | - | - | <10 | 34 | 75.5 | 45 |
| Philippines | 16 | 100 | 16 | - | - | <10 |
| Romania | - | - | <10 | 100 | 100 | 100 |
| Saudi Arabia | - | - | <10 | 68 | 68.7 | 99 |
| South Africa | 1433 | 71.1 | 2015 | 18306 | 69.0 | 26512 |
| Spain | 11 | 64.7 | 17 | 37 | 66.1 | 56 |
| Turkey | 27 | 100 | 27 | |||
| USA | 0 | 0 | 11 | - | - | <10 |
Percentages of patients with at least one comorbidity by country and study period.
Only countries with at least 10 patients in each study period are included.
| Before 10% | After 90% | |||
|---|---|---|---|---|
| Country | % with one or more comorbidities | Total | % with one or more comorbidities | Total |
| Brazil | 78.6 | 14 | 81.8 | 33 |
| Canada | 76.7 | 60 | 74.2 | 760 |
| India | 44.9 | 89 | 56.9 | 209 |
| Malaysia | 64.3 | 42 | 72.7 | 11 |
| Nepal | 46.2 | 197 | 55.4 | 204 |
| Netherlands | 86.7 | 60 | 78.5 | 65 |
| South Africa | 53.9 | 3170 | 44.7 | 37412 |
| Spain | 76.2 | 21 | 76.8 | 56 |
| United Kingdom | 86.5 | 11820 | 84.6 | 21501 |
Percentages of patients with at least one symptom by country and study period.
Only countries with at least 10 patients in each study period are included.
| Before 10% | After 90% | |||
|---|---|---|---|---|
| Country | % with one or more symptoms | Total | % with one or more symptoms | Total |
| Brazil | 100.0 | 14 | 100.0 | 32 |
| Canada | 91.7 | 60 | 91.6 | 754 |
| India | 28.1 | 89 | 18.6 | 210 |
| Malaysia | 64.3 | 42 | 90.9 | 11 |
| Nepal | 97.0 | 197 | 86.3 | 204 |
| Netherlands | 96.7 | 60 | 96.9 | 65 |
| Spain | 100.0 | 21 | 87.5 | 56 |
| United Kingdom | 97.4 | 11104 | 89.3 | 16157 |
Medians (interquartile ranges [Q1 - Q3]) of time from admission or disease onset to death by study period and country.
Only countries with 10 or more observations in both study periods are presented.
| pre-Omicron period | Omicron period | ||||||
|---|---|---|---|---|---|---|---|
| Country | Median | Q1 | Q3 | Median | Q1 | Q3 | |
| Canada | 10 | 6 | 21 | 10 | 5 | 18 | |
| United Kingdom | 11 | 6 | 19 | 11 | 6 | 19 | |
| India | 6 | 3 | 8 | 7 | 3 | 12 | |
| Nepal | 6 | 5 | 12 | 4 | 2 | 8 | |
| South Africa | 6 | 2 | 12 | 5 | 2 | 10 | |
Survival models.
Results of a Cox proportional hazards model, stratified by country, on time to death in the first 28 days since hospital admission or onset of symptoms, which happened latest, are shown in the Hazard ratio column. For this analysis, if follow-up duration was longer than 28 days, it was set to 28 days, and patients who were discharged were censored on the day of discharge. The assumption of proportional hazards was violated for the variable on previous vaccination; for this reason, the model was also stratified by this variable. An alternative analysis assumed that patients discharged from hospital were censored on day 28; in this analysis, the hazard ratio for the variable corresponding to study period was 0.68 (0.63–0.74); for this model, the proportional hazards assumption did not hold for the study period variable. We also fit a competing risk model, with hospital discharge as competing event; estimates from this model are presented in the Subhazard ratio column. In this model, previous COVID-19 vaccination was included as a covariate (subhazard ratio 0.55, 95% CI 0.52–0.59). We also fit a competing risk model using only data from the six countries included in Figures 3—5 and that included country as a dummy variable; in this model, the subhazard ratio for the Omicron period variable was 0.68 95% CI (0.63–0.74).
| Hazard ratio | Subhazard ratio | ||
|---|---|---|---|
| Variables | |||
| Omicron period | 0.77 (0.71–0.84) | 0.79 (0.73–0.84) | |
| Sex (male) | 1.24 (1.17–1.32) | 1.32 (1.24–1.40) | |
| Age | |||
| Older than 60 years | Reference | ||
| Aged between 18 and 60 years | 0.41 (0.38–0.44) | 0.26 (0.24–0.28) | |
| Younger than 18 years | 0.13 (0.11–0.17) | 0.06 (0.04–0.07) |
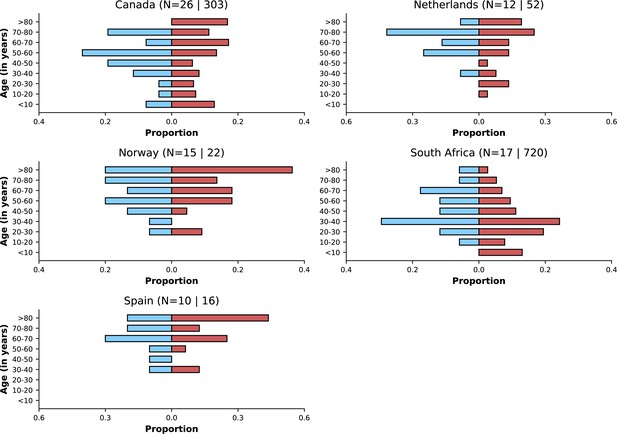
Distribution of infections with individual-level variant information by country and variant.
Only countries with at least 10 observations for Delta and Omicron variants are listed. Note that other countries had limited numbers for both or one of the two variants.
| Country | Delta | Omicron |
|---|---|---|
| Canada | 26 | 303 |
| Netherlands | 12 | 52 |
| Norway | 15 | 22 |
| South Africa | 17 | 720 |
| Spain | 10 | 16 |





