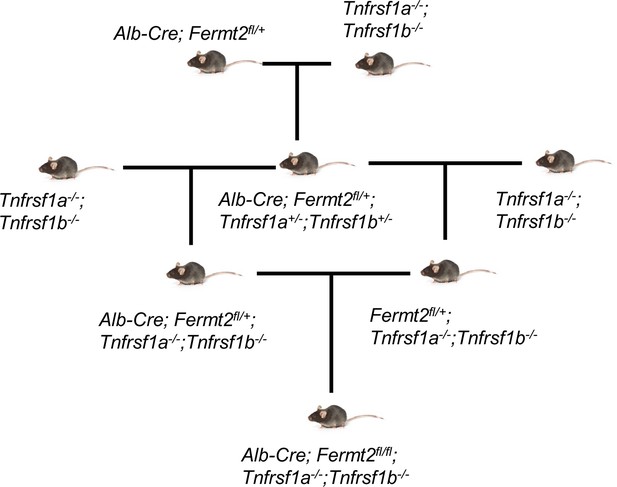
Kindlin-2 inhibits TNF/NF-κB-Caspase 8 pathway in hepatocytes to maintain liver development and function
Figures
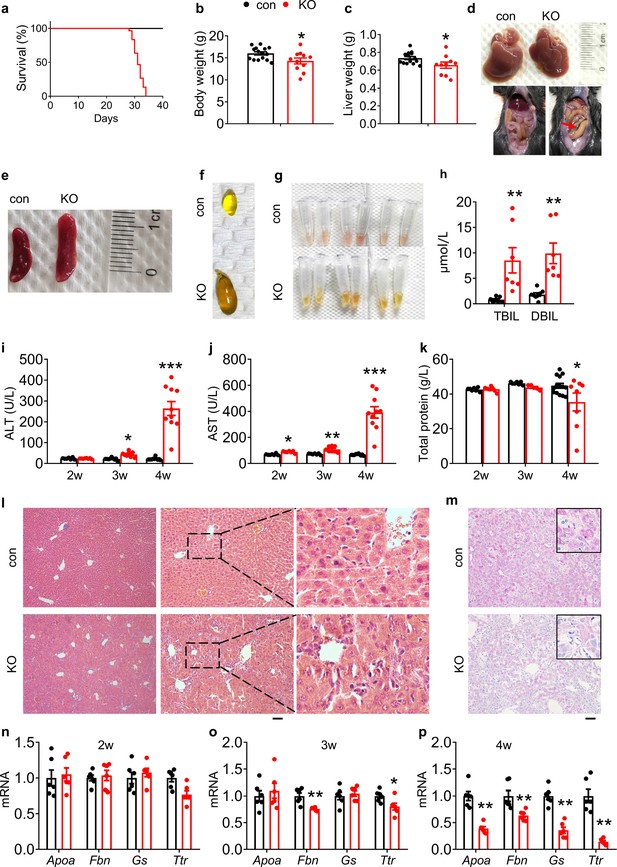
Kindlin-2 loss in hepatocytes causes liver injury and premature death in mice.
(a) Survival curve of control and KO mice (N=30 mice/group). (b) Body weight of 4-week-old control and KO mice (N=15 for control mice, N=11 for KO mice). (c) Liver weight of 4-week-old control and KO mice (N=14 for control mice, N=10 mice for KO mice). (d) Livers and gross appearance of 4-week-old control and KO mice. Red arrow indicates massive ascites in KO. (e) Spleens from 4-week-old control and KO mice. (f) Gallbladder from 4-week-old control and KO mice. (g) Serum appearance from 4-week-old control and KO mice. (h) Serum total bilirubin (TBIL) and direct bilirubin (DBIL) levels. (N=8 for control mice, N=7 mice for KO mice). (i, j) Serum aminotransferase (alanine transaminase [ALT] and aspartate transaminase [AST]) activity in 2-, 3-, and 4-week-old control and KO mice. (k) Serum total protein levels in 2-, 3-, and 4-week-old control and KO mice. (l) Hematoxylin and eosin staining of liver sections of 4-week-old control and KO mice. Scale bars,100 μm. (m) Periodic acid-Schiff staining for liver glycogen at 4 weeks of age. Scale bars,100 μm. (n–p) Quantitative real-time RT-PCR analysis of expression of liver genes in 2-, 3-, and 4-week-old control and KO mice (N=6 mice/group). The results are shown as means ± SEM. *p<0.05, **p<0.01, vs control.
-
Figure 1—source data 1
Raw data related to Figure 1.
- https://cdn.elifesciences.org/articles/81792/elife-81792-fig1-data1-v2.zip
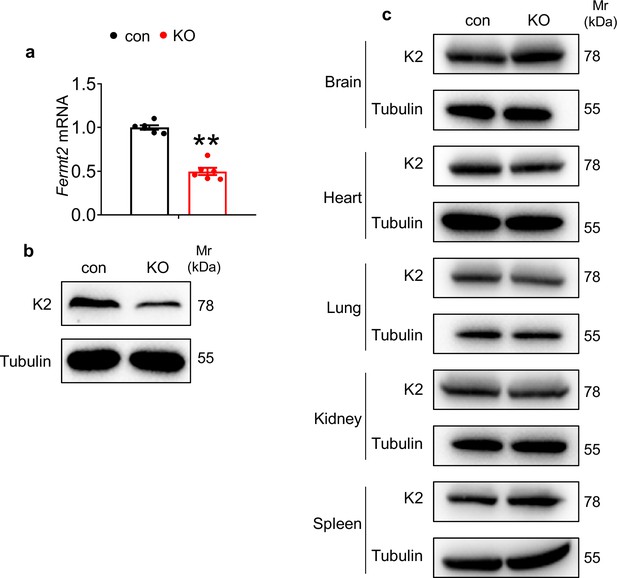
Deletion of Kindlin-2 in hepatocyte resulted in systemic dysfunction.
(a) Quantitative real-time RT-PCR (qRT-PCR) analysis. RNAs isolated from liver tissues of 4-week-old control and KO mice were subjected to qRT-PCR analysis (N=6 mice/group). (b) Western blotting. Liver extracts from 4-week-old control and KO mice were subjected to western blotting analysis for expression of Kindlin-2. Tubulin was used as a loading control. (c) Western blotting. Protein expression of Kindlin-2 was determined by western blotting in the indicated tissues of 4-week-old control and KO mice. The results are shown as means ± SEM. **p<0.01 vs control.
-
Figure 1—figure supplement 1—source data 1
Raw data related to Figure 1—figure supplement 1.
- https://cdn.elifesciences.org/articles/81792/elife-81792-fig1-figsupp1-data1-v2.zip
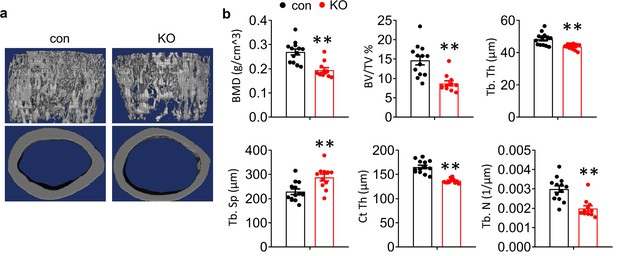
Deletion of Kindlin-2 in hepatocyte resulted in osteoporosis.
(a) Three-dimensional reconstruction from micro-computerized tomography scans of femurs from con and KO mice. (b) Bone histomorphometric analyses. Bone mineral density (BMD), bone volume fraction (BV/TV), trabecular number (Tb. N), trabecular thickness (Tb. Th), trabecular separation (Tb. Sp) and cortical bone thickness (Ct.Th). (N=13 for control mice, N=11 for KO mice). The results are shown as means ± SEM. **p<0.01 vs control.
-
Figure 1—figure supplement 2—source data 1
Raw data related to Figure 1—figure supplement 2.
- https://cdn.elifesciences.org/articles/81792/elife-81792-fig1-figsupp2-data1-v2.zip
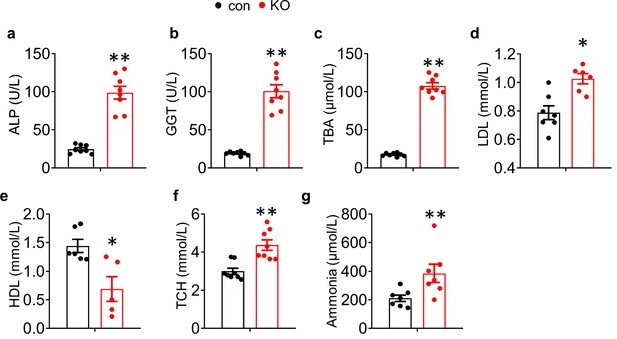
Metabolic parameters were assessed on 4-week-old mice.
(a) ALP: alkaline phosphatase, (N=8 mice/group). (b) GGT: γ -glutamyl transferase, (N=8 mice/group). (c) TBA: total bile acid, (N=8 mice/group). (d) LDL: low density lipoprotein, (N=7 for control mice, N=6 for KO mice). (e) HDL: high density lipoprotein, (N=6 for control mice, N=5 for KO mice). (f) TCH: total cholesterol, (N=8 mice/group). (g) Ammonia level, (N=7 mice/group). The results are shown as means ± SEM. *p<0.05, **p<0.01 vs control.
-
Figure 1—figure supplement 3—source data 1
Raw data related to Figure 1—figure supplement 3.
- https://cdn.elifesciences.org/articles/81792/elife-81792-fig1-figsupp3-data1-v2.zip
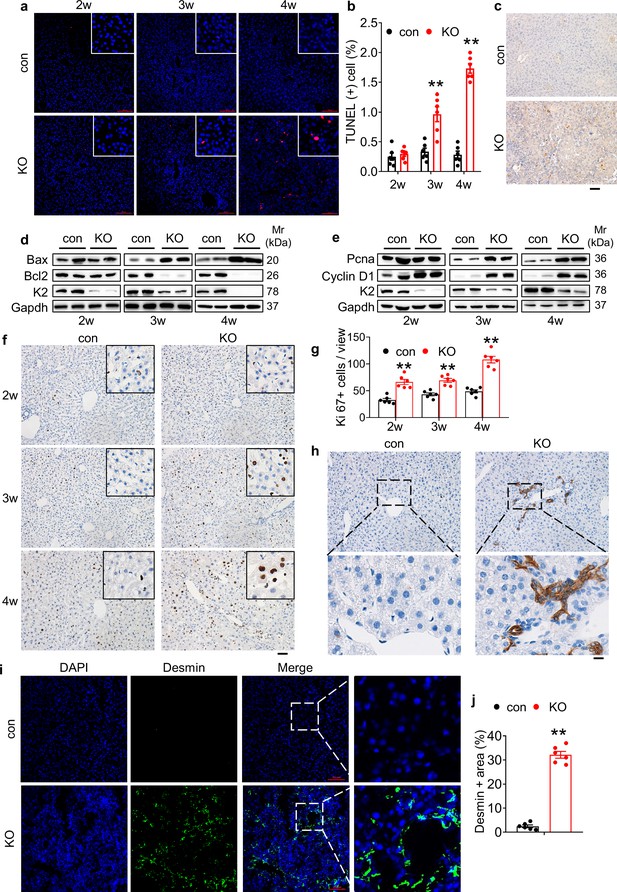
Kindlin-2 loss causes dramatic hepatocyte apoptosis followed by enhanced proliferation of both biliary cells and hepatic stellate cells.
(a) TUNEL staining. Scale bars,100 μm. (b) Quantification of TUNEL-positive cells from 6 different fields from 6 mice per group. (c) Immunohistochemistry (IHC) staining for Caspase 3 was performed on paraffin liver sections of 4-week-old control and KO mice. Scale bars,100 μm. (d, e) Western blotting. Liver tissue lysates from 2-, 3-, and 4-week-old control and KO mice were subjected to western blotting analysis for expression of the indicated proteins. Gapdh was used as a loading control. (f, g) Ki67 staining. Liver sections from of 2-, 3-, and 4-week-old control and KO mice were subjected to Ki67 staining. Scale bars,100 μm. Quantification of (f). (h) IHC staining. Liver sections from 4-week-old control and KO mice were subjected to IHC staining for CK19. Scale bars,100 μm. Representative images showing a dramatic increase in the number CK19-positive cells in KO liver. (i, j) Immunofluorescence (IF) staining. Scale bars,100 μm. Liver sections from 4-week-old control and KO mice were subjected to IF staining for Desmin, a marker for hepatic stellate cells. Representative images showing high expression of Desmin in KO liver. Quantification of (i) from six mice per group. The results are shown as means ± SEM. *p<0.05, **p<0.01, vs control.
-
Figure 2—source data 1
Raw data related to Figure 2.
- https://cdn.elifesciences.org/articles/81792/elife-81792-fig2-data1-v2.zip
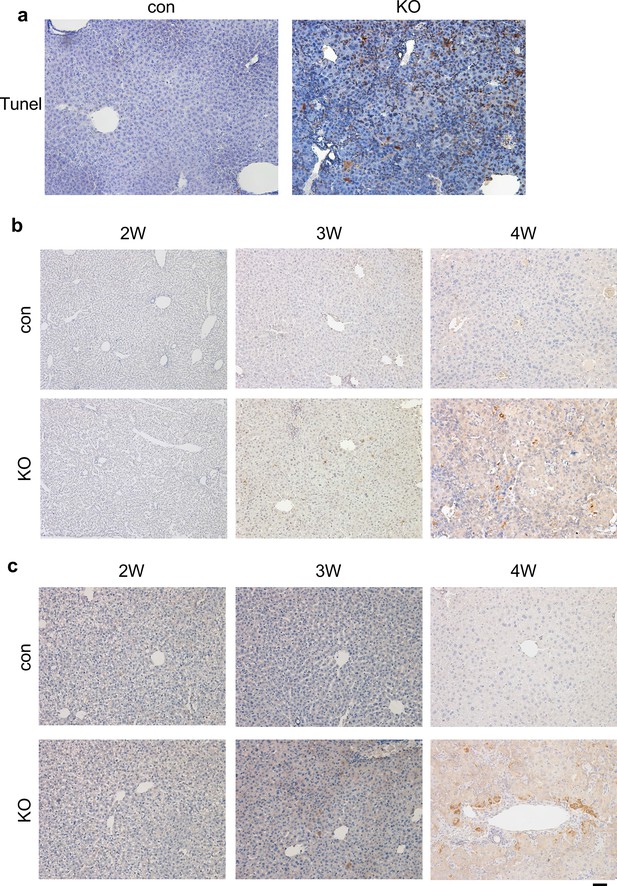
Loss of Kindlin-2 resulted in increased apoptosis.
(a) TUNEL staining shows increased number of apoptotic (yellow stain) cells in 4 weeks old KO liver. (b, c) Immunohistochemistry staining of different time liver sections from mice of the indicated genotypes for expression of active Caspase 3 (b) and p65 (c). Scale bars,100 μm.
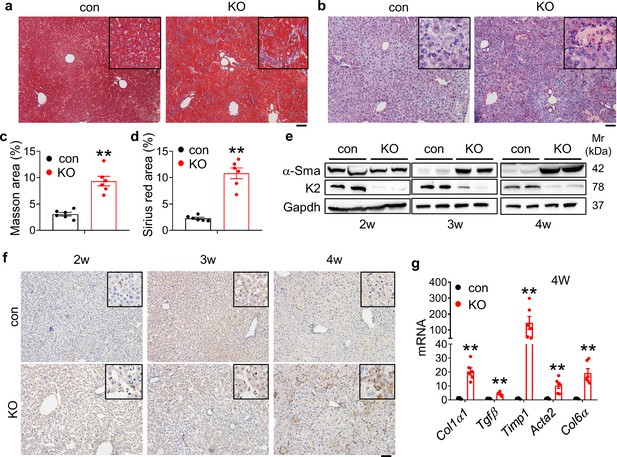
Kindlin-2 loss promotes collagen extracellular matrix deposition and fibrosis in liver.
(a) Masson’s trichrome staining of liver sections of 4-week-old control and KO mice. Scale bars,100 μm. (b) Sirius red staining. Fibrillar collagen deposition in liver sections of 4-week-old control and KO mice was determined by Sirius red staining. Scale bars,100 μm. (c, d) Quantification of (a) and (b) from six mice per group. (e) Western blotting. Liver tissue extracts from 2-, 3-, and 4-week-old control and KO mice were subjected to western blotting for expression of α-Sma and Kindlin-2. Gapdh was used as loading control. (f) Immunohistochemistry (IHC) staining. Liver sections from 2-, 3-, and 4-week-old control and KO mice were subjected to IHC staining using an antibody against α-Sma. Scale bars,100 μm. (g) Quantitative real-time RT-PCR (qRT-PCR) analysis. RNAs isolated from liver tissues of 4-week-old control and KO mice were subjected to qRT-PCR analysis for expression of fibrosis-related genes. (N=6 mice/group). The results are shown as means ± SEM. *p<0.05, **p<0.01, vs control.
-
Figure 3—source data 1
Raw data related to Figure 3.
- https://cdn.elifesciences.org/articles/81792/elife-81792-fig3-data1-v2.zip
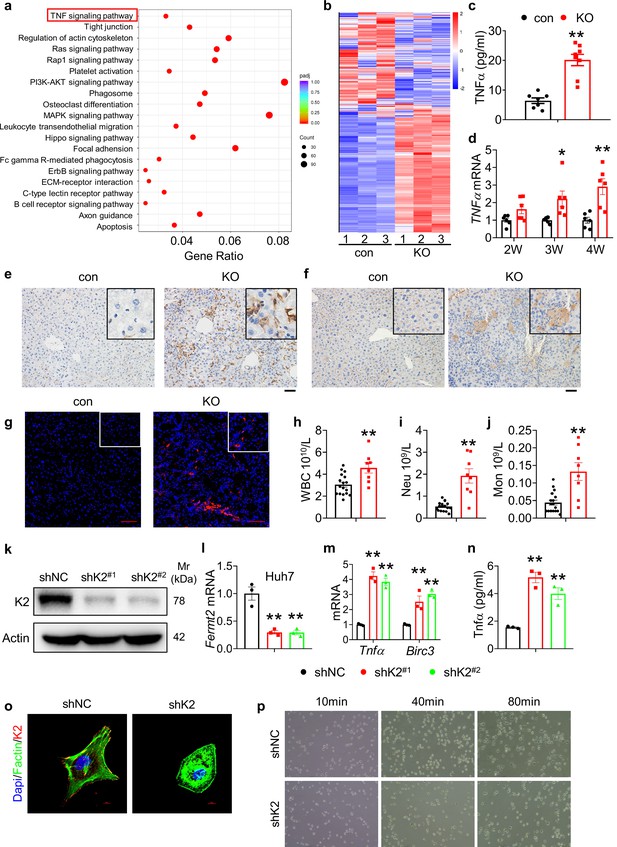
Kindlin-2 loss activates the TNF/NF-κB signaling pathway.
(a) Kyoto Encyclopedia of Genes and Genome analysis showing the up-regulated pathways in the 4-week-old KO mice versus control mice. (b) Heatmap represents genes with >1.5 fold upregulation or >1.5 fold downregulation in K2-deficient liver compared with control liver. (c) Serum from 4-week-old control and KO mice was subjected to ELISA for Tnfα protein (N=7–8 mice/group). (d) RNAs isolated from liver tissues of 2-, 3-, and 4-week-old control and KO mice were subjected to quantitative real-time RT-PCR (qRT-PCR) analyses for Tnfα genes (N=6 mice/group). (e) 4-week-old control and KO mouse liver sections were subjected to immunohistochemistry (IHC) staining using an anti-F4/80 antibody to determine macrophage infiltration. Scale bars,100 μm. (f) 4-week-old control and KO mouse liver sections were subjected to IHC staining using an anti-CD19 antibody to determine B cell infiltration. Scale bars,100 μm. (g) 4-week-old control and KO mouse liver sections were subjected to immunofluorescence (IF) staining using an anti-Ly6G antibody to determine neutrophils infiltration. Scale bars,100 μm. (h–j) Complete blood count was assessed on 4-week-old mice. WBC: white blood cell, Neu: neutrophil, Mon: monocyte. N=17 for control mice and N=8 for KO mice. (k, l) Kindlin-2 knockdown. qRT-PCR analyses and western blotting were performed to detect Kindlin-2 expression in Huh7 cells treated with lentiviruses-expressed control shRNA (shNC) and two different Fermt2 shRNAs (shK2(#1), shK2(#2)). (m) qRT-PCR analysis of Tnfα and BircI3 mRNAs in shNC- and shK2-treated Huh7 cells. (n) ELISA. The levels of Tnfα protein in media of shNC- and shK2-treated Huh7 cultures were assayed using an ELISA kit. (o) (IF) staining for DAPI, F-actin, and Kindlin-2 in shNC and shK2 cells. (p) Representative images of shNC and shK2 cells spreading and attachment on glass surface at the indicated times after seeding. Each sample was tested at least in triplicate independent cell preparations.The results are shown as means ± SEM. *p<0.05, **p<0.01, vs control.
-
Figure 4—source data 1
Raw data related to Figure 4.
- https://cdn.elifesciences.org/articles/81792/elife-81792-fig4-data1-v2.zip
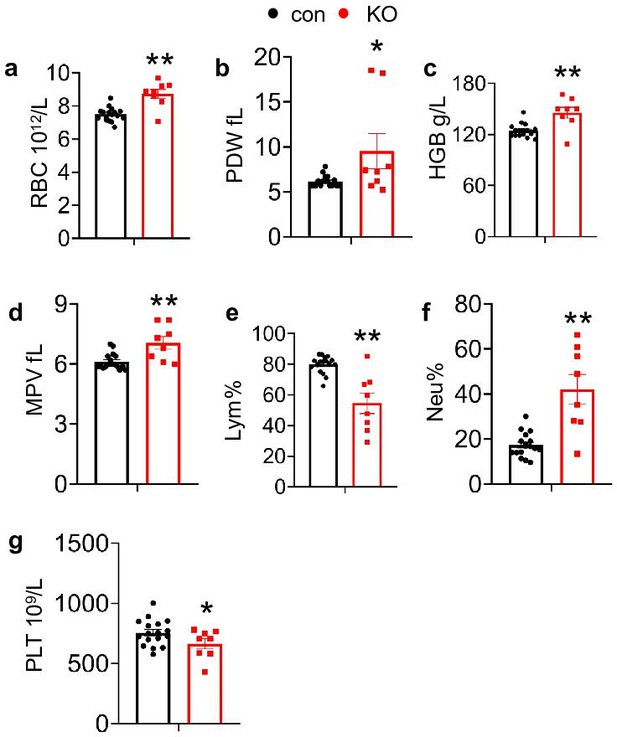
Complete blood count was assessed on 4-week-old mice.
(a) RBC: red blood cell, (b) PDW: platelet distribution width, (c) HGB: hemoglobin, (d) MPV: mean platelet volume, (e) Lym: lymphocyte, (f) Neu: neutrophil, (g) PLT: blood platelet. (N=17 for control mice and N=8 for KO mice). The results are shown as means ± SEM. *p<0.05, **p<0.01 vs control.
-
Figure 4—figure supplement 1—source data 1
Raw data related to Figure 4—figure supplement 1.
- https://cdn.elifesciences.org/articles/81792/elife-81792-fig4-figsupp1-data1-v2.zip
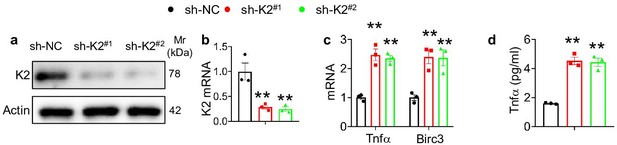
Kindlin-2 loss in HepG2 cells resulted in upregulation of TNF signaling pathway.
(a, b) Kindlin-2 knockdown. qPCR analyses and western blotting were performed to detect Kindlin-2 expression in HepG2 cells treated with lentiviruses-expressed control shRNA (shNC) and two different Fermt2 shRNAs (sh-K2#1, sh-K2#2). (c) qPCR analysis of Tnfα and BircI3 mRNAs in shNC- and shK2-treated HepG2 cells. (d) ELISA. The levels of Tnfα protein in media of shNC- and shK2-treated HepG2 cultures were assayed using an ELISA kit. The results are shown as means ± SEM. *p<0.05, **p<0.01 vs shNC.
-
Figure 4—figure supplement 2—source data 1
Raw data related to Figure 4—figure supplement 2.
- https://cdn.elifesciences.org/articles/81792/elife-81792-fig4-figsupp2-data1-v2.zip
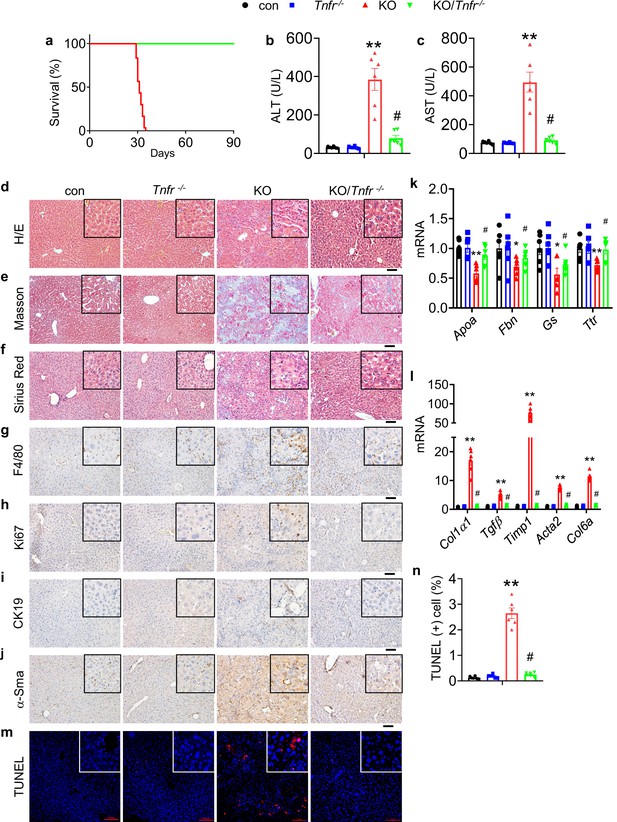
Global Tnfr genes deletion rescues the liver injury and lethality induced by Kindlin-2 loss.
(a) Survival curve of KO and KO/Tnfr-/- mice. N=30 mice in KO group, N=33 for KO/Tnfr-/- group. (b, c) Serum alanine transaminase (ALT) and aspartate transaminase (AST) levels in control, KO, Tnfr-/-, and KO/Tnfr-/- mice. N=6 mice/group. (d-f) Liver histology. Liver sections from mice of the indicated genotypes were subjected to hematoxylin and eosin (H/E) staining (d), Masson’s trichrome staining (e) and Sirius Red staining (f). Scale bars,100 μm. (g–j) Immunohistochemistry staining of liver sections from mice of the indicated genotypes for expression of F4/80 (g), Ki67 (h), CK19 (i), and α-Sma (j). Scale bars,100 μm. (k, l) Quantitative real-time RT-PCR (qRT-PCR) analysis. RNAs isolated from liver tissues of the indicated genotypes were subjected to qRT-PCR analysis for expression of the indicated genes. N=6 mice/group. (m, n) TUNEL staining of the indicated genotypes and quantification. N=6 mice/group. Scale bars,100 μm. The results are shown as means ± SEM. *p<0.05, **p<0.01, vs control. #p<0.05, vs KO.
-
Figure 5—source data 1
Raw data related to Figure 5.
- https://cdn.elifesciences.org/articles/81792/elife-81792-fig5-data1-v2.zip
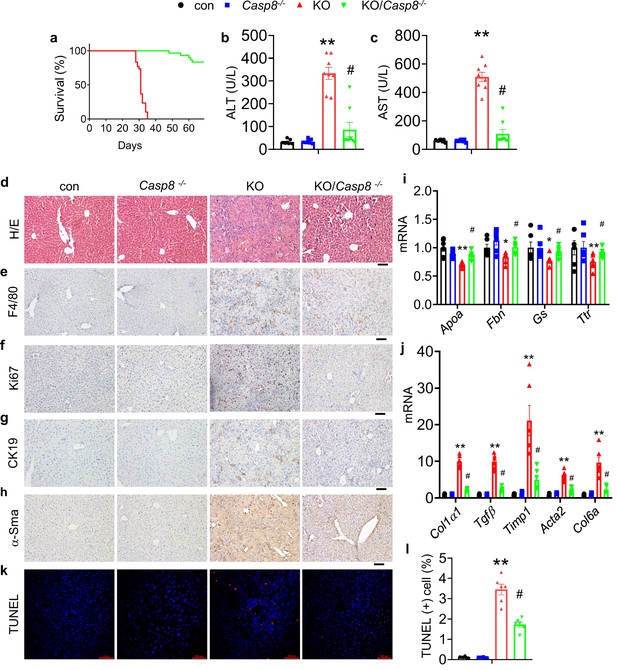
Caspase 8 deletion in hepatocytes rescues the liver lesions and lethality caused by Kindlin-2 deficiency.
(a) Survival curve of KO and KO/Casp8-/- mice. N=30 mice/group. (b, c) Serum alanine transaminase (ALT) and aspartate transaminase (AST) levels in control, KO, Casp8-/-, and KO/Casp8-/- mice. N=8 mice/group. (d) Liver histology. Liver sections from mice of the indicated genotypes were subjected to hematoxylin and eosin (H/E) staining, Scale bars,100 μm. (e–h) Immunohistochemistry staining of liver sections from mice of the indicated genotypes for expression of F4/80 (e), Ki67 (f), CK19 (g), and α-Sma (h). Scale bars,100 μm. (i, j) Quantitative real-time RT-PCR (qRT-PCR) analysis. RNAs isolated from liver tissues of the indicated genotypes were subjected to qRT-PCR analysis for expression of the indicated genes. N=6 mice/group. (k, l) TUNEL staining of the indicated genotypes and quantification. N=6 mice/group. Scale bars,100 μm. The results are shown as means ± SEM. *p<0.05, **p<0.01, vs control. #p<0.05, vs KO.
-
Figure 6—source data 1
Raw data related to Figure 6.
- https://cdn.elifesciences.org/articles/81792/elife-81792-fig6-data1-v2.zip
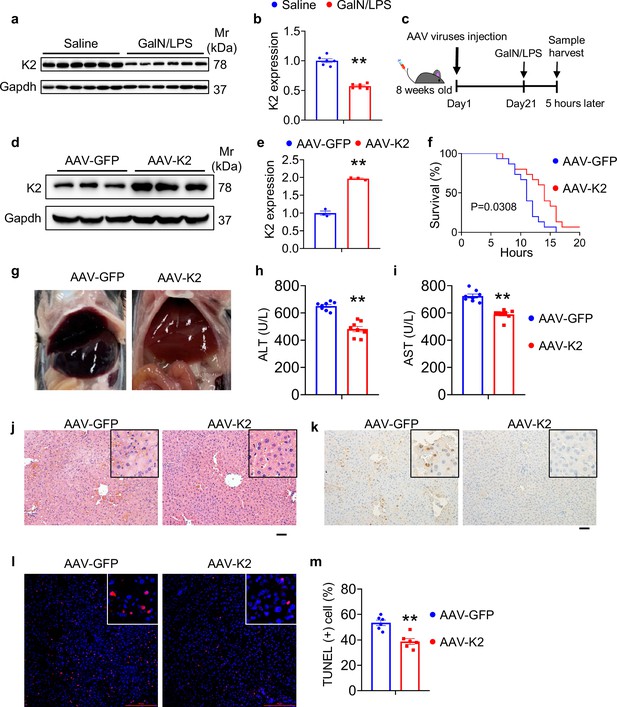
Overexpression of Kindlin-2 ameliorates D-galactosamine/lipopolysaccharide (D-GalN/LPS)-induced liver injury and death.
(a, b) Eight-week-old C57BL/6 mice were intraperitoneally injected with D-GaIN/LPS (GalN, 700 mg/kg body weight, LPS, 3 mg/kg body weight) or PBS as controls for 5 hr. Liver tissues were collected and subjected to western blotting for expression of Kindlin-2. Quantitative data (a). N=6 mice/group. (c) Experimental design. (d, e) Western blotting. Eight-week-old C57BL/6 mice were first injected via tail vein with adeno-associated virus 8 (AAV8) (2×1011 particles/mouse) expressing Kindlin-2 (AAV8-K2) or GFP (AAV8-GFP). After 21 days, mice were then treated with D-GaIN/LPS for 5 hr. Protein extracts were then prepared from livers and subjected to western blotting for expression of Kindlin-2. Quantitative data (d). Gapdh was used as a loading control. N=3 mice per group. (f) Survival curve. Mice were treated as in (c), followed by observation for death. N=15 mice per group. (g) Gross liver appearance. Mice were treated as in (c). (h, i) ELISA assays. Serum ALT (h) and AST (i). Mice were treated as in (c). N=8 mice per group. (j) Hematoxylin and eosin staining in liver sections. Mice were treated as in (c). (k) Immunohistochemistry staining of Caspase-3 in liver sections. Mice were treated as in (c). (l, m) TUNEL staining of liver sections. Quantitation data (m). Mice were treated as in (c). N=6 mice per group. The results are shown as means ± SEM. **p<0.01, vs AAV-GFP.
-
Figure 7—source data 1
Raw data related to Figure 7.
- https://cdn.elifesciences.org/articles/81792/elife-81792-fig7-data1-v2.zip
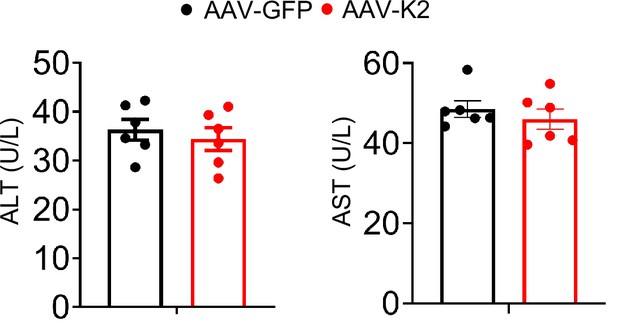
Serum alanine transaminase (ALT) and aspartate transaminase (AST).
Eight-week-old C57BL/6 mice were first injected via tail vein with adeno-associated virus 8 (AAV8) (2×1011 particles/mouse) expressing Kindlin-2 (AAV8-K2) or GFP (AAV8-GFP). After 2 months, mice were sacrificed, and serum were collected to detect ALT and AST (N=6 mice/group).
-
Figure 7—figure supplement 1—source data 1
Raw data related to Figure 7—figure supplement 1.
- https://cdn.elifesciences.org/articles/81792/elife-81792-fig7-figsupp1-data1-v2.zip
Additional files
-
Supplementary file 1
The antibody information and primer sequences.
(a) Antibody information. (b) Primer information for mouse. (c) primer information for human.
- https://cdn.elifesciences.org/articles/81792/elife-81792-supp1-v2.docx
-
MDAR checklist
- https://cdn.elifesciences.org/articles/81792/elife-81792-mdarchecklist1-v2.docx