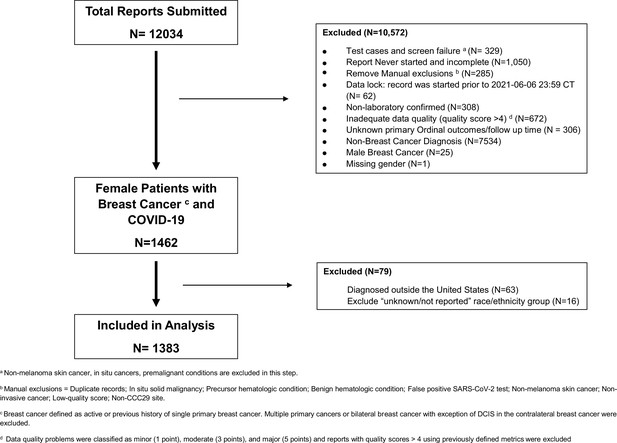
Clinical characteristics, racial inequities, and outcomes in patients with breast cancer and COVID-19: A COVID-19 and cancer consortium (CCC19) cohort study
Figures
Tables
Baseline characteristics by race/ethnicity.
| NHW | Black | Hispanic | AAPI | Others | All | |
|---|---|---|---|---|---|---|
| (n=736, 53%) | (n=289, 21%) | (n=235, 17%) | (n=45, 3%) | (n=78, 6%) | (n=1383, 100%) | |
| Median age, years* [IQR] | 64 (54–76) | 61 (52–69) | 53 (46–62) | 54 (43–73) | 62 (53–71) | 61 (51–72) |
| Median follow-up, days [IQR] | 90 (30–135) | 90 (30–180) | 90 (30–135) | 42 (21–90) | 70 (30–180) | 90 (30–135) |
| Smoking status | ||||||
| Never | 460 (62%) | 186 (64%) | 180 (77%) | 35 (78%) | 50 (64%) | 911 (66%) |
| Current or former | 261 (35%) | 95 (33%) | 53 (23%) | 8 (18%) | 25 (32%) | 442 (32%) |
| Missing/unknown | 15 (2%) | 8 (3%) | 2 (1%) | 2 (4%) | 3 (4%) | 30 (2%) |
| Obesity | ||||||
| No | 421 (57%) | 133 (46%) | 116 (49%) | 32 (71%) | 45 (58%) | 747 (54%) |
| Yes | 308 (42%) | 156 (54%) | 116 (49%) | 13 (29%) | 33 (42%) | 626 (45%) |
| Missing/unknown | 7 (1%) | 0 (0%) | 3 (1%) | 0 (0%) | 0 (0%) | 10 (1%) |
| Comorbidities† | ||||||
| Cardiovascular | 179 (24%) | 60 (21%) | 14 (6%) | 7 (16%) | 11 (14%) | 271 (20%) |
| Pulmonary | 125 (17%) | 65 (22%) | 33 (14%) | <5 (<11%) | 7 (9%) | 234 (17%) |
| Renal disease | 66 (9%) | 31 (11%) | 13 (6%) | <5 (<11%) | <5 (<6%) | 115 (8%) |
| Diabetes mellitus | 127 (17%) | 98 (34%) | 51 (22%) | 10 (22%) | 20 (26%) | 306 (22%) |
| Missing/unknown | 9 (1%) | 1 (<1%) | 5 (2%) | 0 (0%) | 0 (0%) | 15 (1%) |
| ECOG performance status | ||||||
| 0 | 314 (43%) | 130 (45%) | 123 (52%) | 18 (40%) | 32 (41%) | 617 (45%) |
| 1 | 135 (18%) | 72 (25%) | 48 (20%) | 10 (22%) | 16 (21%) | 281 (20%) |
| 2+ | 69 (9%) | 33 (11%) | 15 (6%) | 5 (11%) | 5 (6%) | 127 (9%) |
| Unknown | 218 (30%) | 53 (18%) | 49 (21%) | 12 (27%) | 25 (32%) | 357 (26%) |
| Missing | 0 (0%) | 1 (<1%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (<1%) |
| Region | ||||||
| Northeast | 247 (34%) | 101 (35%) | 106 (45%) | 12 (27%) | 26 (33%) | 492 (36%) |
| Midwest | 239 (32%) | 110 (38%) | 23 (10%) | 8 (18%) | 12 (15%) | 392 (28%) |
| South | 116 (16%) | 58 (20%) | 27 (11%) | X* | 14 (18%) | 218 (16%) |
| West | 128 (17%) | 16 (6%) | 77 (33%) | 22 (49%) | 24 (31%) | 267 (19%) |
| Undesignated | 6 (1%) | 4 (1%) | 2 (1%) | 3 (7%)* | 2 (3%) | 14 (1%) |
| Month/year of COVID-19 diagnosis | ||||||
| Jan-Apr 2020 | 140 (19%) | 74 (26%) | 41 (17%) | 8 (18%) | 20 (26%) | 283 (20%) |
| May-Aug 2020 | 279 (38%) | 141 (49%) | 101 (43%) | 24 (53%) | 30 (38%) | 575 (42%) |
| Sept-Dec 2020 | 197 (27%) | 42 (15%) | 50 (21%) | 5 (11%) | 16 (21%) | 310 (22%) |
| Jan-Jun 2021 | 118 (16%) | 32 (11%) | 41 (17%) | 7 (16%) | 12 (15%) | 210 (15%) |
| Missing/unknown | 2 (<1%) | 0 (0%) | 2 (1%) | 1 (2%) | 0 (0%) | 5 (<1%) |
| Area of patient residence | ||||||
| Urban | 193 (26%) | 136 (47%) | 124 (53%) | 13 (29%) | 30 (38%) | 496 (36%) |
| Suburban | 315 (43%) | 77 (27%) | 65 (28%) | 17 (38%) | 31 (40%) | 505 (37%) |
| Rural | 81 (11%) | 7 (2%) | 9 (4%) | X* | 0 (0%) | 98 (7%) |
| Missing/unknown | 147 (20%) | 69 (24%) | 37 (16%) | 15 (33%)* | 17 (22%) | 284 (21%) |
| Treatment center characteristics | ||||||
| Academic medical center | 123 (17%) | 102 (35%) | 43 (18%) | 7 (16%) | 11 (14%) | 286 (21%) |
| Community practice | 238 (32%) | 51 (18%) | 44 (19%) | X* | 23 (29%) | 359 (26%) |
| Tertiary care center | 375 (51%) | 136 (47%) | 147 (63%) | 35 (78%) | 44 (56%) | 737 (53%) |
| Missing/unknown | 0 (0%) | 0 (0%) | 1 (<1%) | 3 (7%)* | 0 (0%) | 1 (<1%) |
| Receptor status | ||||||
| HR+/HER2- | 419 (57%) | 135 (47%) | 102 (43%) | 22 (49%) | 43 (55%) | 721 (52%) |
| HR+/HER2+ | 102 (14%) | 35 (12%) | 43 (18%) | 7 (16%) | 9 (12%) | 196 (14%) |
| HR-/HER2+ | 46 (6%) | 28 (10%) | 32 (14%) | X* | X* | 111 (8%) |
| Triple negative | 57 (8%) | 54 (19%) | 35 (15%) | 5 (11%) | 7 (9%) | 158 (11%) |
| Missing/unknown | 112 (15%) | 37 (13%) | 23 (10%) | 11 (24%) | 19 (24%)* | 197 (14%) |
| Cancer status | ||||||
| Remission/NED, >5 years | 247 (34%) | 76 (26%) | 23 (10%) | 9 (20%) | 20 (26%) | 375 (27%) |
| Remission/NED, <5 years | 234 (32%) | 100 (35%) | 77 (33%) | 11 (24%) | 26 (33%) | 448 (32%) |
| Active and responding | 68 (9%) | 35 (12%) | 56 (24%) | X* | 11 (14%) | 173 (13%) |
| Active and stable | 91 (12%) | 28 (10%) | 35 (15%) | 10 (22%) | 5 (6%) | 169 (12%) |
| Active and progressing | 41 (6%) | 27 (9%) | 20 (9%) | 6 (13%) | X* | 97 (7%) |
| Unknown | 48 (7%) | 19 (7%) | 22 (9%) | 6 (13%)* | 15 (19%)* | 104 (8%) |
| Missing | 7 (1%) | 4 (1%) | 2 (1%) | 3 (7%) | 1 (1%) | 17 (1%) |
| Timing of anti-cancer therapy | ||||||
| Never/after COVID-19 | 24 (3%) | 10 (3%) | 7 (3%) | X* | 7 (9%) | 50 (4%) |
| 0–4 weeks | 364 (49%) | 135 (47%) | 158 (67%) | 25 (56%) | 39 (50%) | 721 (52%) |
| 1–3 months | 26 (4%) | 20 (7%) | 19 (8%) | 0 (0%) | X* | 69 (5%) |
| >3 months | 303 (41%) | 118 (41%) | 45 (19%) | 18 (40%) | 24 (31%) | 508 (37%) |
| Missing/unknown | 19 (3%) | 6 (2%) | 6 (3%) | 2 (4%)* | 8 (10%)* | 35 (3%) |
| Modality of active anti-cancer therapy‡, § | ||||||
| None | 333 (45%) | 127 (44%) | 53 (23%) | 20 (44%) | 30 (38%) | 563 (41%) |
| Chemotherapy | 117 (16%) | 68 (24%) | 88 (37%) | 11 (24%) | 14 (18%) | 298 (22%) |
| Targeted therapy | 112 (15%) | 38 (13%) | 59 (25%) | 8 (18%) | 11 (14%) | 228 (16%) |
| Anti-HER2 therapy | 60 (8%) | 17 (6%) | 36 (15%) | <5 (<11%) | <5 (<6%) | 123 (9%) |
| CDK4/6 inhibitor | 33 (4%) | 12 (4%) | 14 (6%) | <5 (<11%) | <5 (<6%) | 65 (5%) |
| Other¶ | 14 (2%) | 5 (2%) | <5 (<2%) | <5 (<11%) | 0 (0%) | 24 (2%) |
| Endocrine therapy | 283 (38%) | 86 (30%) | 91 (39%) | 15 (33%) | 26 (33%) | 501 (36%) |
| Immunotherapy | 12 (2%) | 8 (3%) | <5 (<2%) | <5 (<11%) | <5 (<6%) | 28 (2%) |
| Local (surgery/radiation) | 80 (11%) | 37 (13%) | 41 (17%) | <5 (<11%) | 9 (12%) | 172 (12%) |
| Other | 13 (2%) | 3 (1%) | 2 (1%) | 0 (0%) | 0 (0%) | 18 (1%) |
| Missing/unknown | 12 (2%) | 7 (2%) | 5 (2%) | 0 (0%) | 5 (6%) | 29 (2%) |
| Severity of COVID-19 | ||||||
| Mild | 535 (73%) | 177 (61%) | 173 (74%) | 28 (62%) | 50 (64%) | 963 (70%) |
| Moderate | 174 (24%) | 97 (34%) | 56 (24%) | 14 (31%) | 21 (27%) | 362 (26%) |
| Severe | 25 (3%) | 15 (5%) | 6 (3%) | X* | 7 (9%) | 56 (4%) |
| Missing/unknown | 2 (<1%) | 0 (0%) | 0 (0%) | 3 (7%)* | 0 (0%) | 2 (<1%) |
-
Variable categories with one to five cases are masked by replacing with N < 5 according to CCC19 policy.
-
*
Cells combined to mask N<5 according to CCC19 low count policy.
-
†
Age was truncated at 90.
-
‡
Percentages could sum to >100% because categories are not mutually exclusive.
-
§
Within 3 months of COVID-19 diagnosis.
-
¶
Therapies other than anti-Her2 therapy or CDK4/6 inhibitor.
Outcomes, clinical complications, and COVID-19 interventions.
| NHW | Black | Hispanic | AAPI | Other | All | |
|---|---|---|---|---|---|---|
| n** (%) | n** (%) | n** (%) | n** (%) | n** (%) | n** (%) | |
| Outcomes | ||||||
| Total all-cause mortality* | 60 (8) | 38 (13) | 12 (5) | <5(<11) | 9 (12) | 123 (9) |
| 30-day all-cause mortality† | 40 (5) | 29 (10) | 8 (3) | <5 (<11) | 8 (10) | 89 (6) |
| Received mechanical ventilation* | 24 (3) | 26 (9) | 11 (5) | <5 (<11) | <5 (<6) | 69 (5) |
| Admitted to an intensive care unit* | 45 (6) | 31 (11) | 18 (8) | 7 (16) | 10 (13) | 111 (8) |
| Admitted to the hospital* | 245 (33) | 137 (47) | 77 (33) | 20 (44) | 33 (42) | 512 (37) |
| Clinical complications | ||||||
| Any cardiovascular complication‡ | 82 (11) | 50 (17) | 30 (13) | 6 (13) | 18 (23) | 186 (14) |
| Any pulmonary complication§ | 170 (23) | 88 (31) | 43 (18) | 12 (27) | 23 (30) | 336 (24) |
| Any gastrointestinal complication¶ | 12 (2) | 7 (2) | <5 (<2) | <5 (<11) | <5 (<7) | 26 (2) |
| Acute kidney injury | 41 (6) | 46 (16) | 11 (5) | 5 (11) | 10 (13) | 113 (8) |
| Multisystem organ failure | 10 (1) | 12 (4) | <5 (<2) | <5 (<11) | <5 (<7) | 29 (2) |
| Superimposed infection | 62 (9) | 42 (15) | 14 (6) | 7 (16) | <5 (<7) | 129 (10) |
| Sepsis | 43 (6) | 24 (8) | 15 (6) | 7 (16) | 12 (16) | 101 (7) |
| Any bleeding | 15 (2) | 7 (2) | <5 (<2) | <5 (<11) | <5 (<7) | 29 (2) |
| Interventions | ||||||
| Remdesivir | 68 (10) | 20 (7) | 15 (7) | 8 (18) | 5 (7) | 116 (9) |
| Hydroxychloroquine | 60 (9) | 41 (15) | 14 (6) | <5 (<11) | 11 (15) | 129 (10) |
| Systemic corticosteroids | 107 (15) | 50 (18) | 31 (14) | 8 (18) | 13 (18) | 209 (16) |
| Other | 112 (16) | 53 (19) | 36 (16) | 11 (25) | 12 (17) | 224 (17) |
| Supplemental oxygen | 173 (24) | 87 (31) | 43 (19) | 14 (31) | 24 (31) | 341 (25) |
-
Variable categories with one to five cases are masked by replacing with N<5 according to CCC19 policy.
-
*
Included in primary outcome.
-
†
Secondary outcome.
-
‡
Cardiovascular complication includes hypotension, myocardial infarction, other cardiac ischemia, atrial fibrillation, ventricular fibrillation, other cardiac arrhythmia, cardiomyopathy, congestive heart failure, pulmonary embolism (PE), deep vein thrombosis (DVT), stroke, thrombosis NOS complication.
-
§
Pulmonary complication includes respiratory failure, pneumonitis, pneumonia, acute respiratory distress syndrome (ARDS), PE, pleural effusion, empyema.
-
¶
Gastrointestinal complication includes acute hepatic injury, ascites, bowel obstruction, bowel perforation, ileus, peritonitis.
-
**
N based on number of patients with non-missing data.
Systemic treatments received within 3 months prior to COVID-19 diagnosis.
| N (%) | |
|---|---|
| Total | 679 (100%) |
| Endocrine therapy alone | 336 (49.5) |
| CDK4/6 inhibitor ± endocrine therapy | 63 (9) |
| Other targeted therapy ± endocrine therapy | 10 (1.5) |
| Anti-HER2 therapy ± endocrine therapy | 78 (11.5) |
| Anti-HER2 therapy + chemotherapy | 48 (7) |
| Single agent chemotherapy ± endocrine therapy | 55 (8) |
| Combination chemotherapy ± endocrine therapy | 60 (9) |
| Immunotherapy ± chemotherapy | 19 (3) |
| Other combination therapies | 10 (1.5) |
Adjusted associations of baseline characteristics with COVID-19 severity outcome.
| COVID-19 severity | |
|---|---|
| OR (95% CI) | |
| Age (per decade) | 1.48 (1.32–1.67) |
| Race (Ref: non-Hispanic White)* | |
| Non-Hispanic Black | 1.74 (1.24–2.45) |
| Hispanic | 1.38 (0.93–2.05) |
| Non-Hispanic AAPI | 3.40 (1.70–6.79) |
| Other | 2.97 (1.71–5.17) |
| Obesity (Ref: No) | 1.20 (0.92–1.57) |
| Cardiovascular comorbidity (Ref: No) | 2.26 (1.63–3.15) |
| Pulmonary comorbidity (Ref: No) | 1.65 (1.20–2.29) |
| Renal disease (Ref: No) | 1.34 (0.86–2.07) |
| Diabetes mellitus (Ref: No) | 2.25 (1.66–3.04) |
| ECOG performance status (Ref: 0) | |
| 1 | 1.74 (1.22–2.48) |
| 2+ | 7.78 (4.83–12.5) |
| Unknown | 2.26 (1.61–3.19) |
| Cancer status (Ref: Remission/NED, >5 years) | |
| Remission or NED, <5 years | 0.91 (0.63–1.33) |
| Active and responding | 1.07 (0.63–1.83) |
| Active and stable | 1.37 (0.82–2.28) |
| Active and progressing | 12.5 (6.89–22.6) |
| Unknown | 1.79 (0.96–3.34) |
| Chemotherapy (Ref: No) | 1.37 (0.91–2.06) |
| Anti-HER2 therapy (Ref: No) | 1.13 (0.67–1.92) |
| CDK 4/6 inhibitor (Ref: No) | 1.21 (0.60–2.42) |
| Other targeted therapies† (Ref: No) | 1.78 (0.69–4.59) |
| Endocrine therapy (Ref: No) | 1.00 (0.73–1.37) |
| Locoregional therapy (Ref: No) | 1.36 (0.88–2.10) |
| Never received cancer treatment (Ref: >3 month) | 0.65 (0.28–1.49) |
| Month/year of COVID-19 diagnosis (Ref: Jan-Apr 2020) | |
| May-Aug 2020 | 0.57 (0.41–0.81) |
| Sept-Dec 2020 | 0.45 (0.30–0.68) |
| Jan-Jun 2021 | 0.57 (0.36–0.89) |
| Region (Ref: Northeast) | |
| Midwest | 0.76 (0.54–1.05) |
| South | 0.76 (0.51–1.13) |
| West | 0.43 (0.29–0.65) |
-
*
Odds ratios greater than 1 indicate higher odds of composite outcome. The p value for evaluating the null hypothesis of equality in odds ratios across race (4 degrees of freedom) was <0.001.
-
†
Therapies other than CDK4/6 inhibitor or anti-HER2 therapy. All variance inflation factors are <1.8 for the model.
Primary outcome.
| Outcome description | Outcome variable name | Outcome values |
|---|---|---|
| Custom ordinal outcome with death at any time | der_ordinal_v1a | 0=not hospitalized; 1=hospitalized; 2=ICU; 3=mechanical ventilation; 4=death at any time |
| Follow-up in days, with some estimation for intervals | der_days_fu | Integer (days) |
Secondary outcome.
| Outcome description | Outcome variable name | Outcome values | Additional Details |
|---|---|---|---|
| Derived dead/alive variable | der_deadbinary | 0=No; 1=Yes; 99=Unknown | |
| Derived variable indicating whether patient has died within 30 days of COVID-19 diagnosis (default = No) | der_dead30 | 0=No; 1=Yes; 99=Unknown | |
| Derived variable indicating whether patients required mechanical ventilation | der_mv | 0=No; 1=Yes; 99=Unknown | |
| Derived variable indicating time in ICU | der_ICU | 0=No; 1=Yes; 99=Unknown | |
| Derived hospitalized/not hospitalized variable | der_hosp | 0=No; 1=Yes; 99=Unknown | |
| Derived cardiovascular complication variable (see additional details) | der_CV_event_v2 (der_any_CV is the variable name in R script) | 0=No; 1=Yes; 99=Unknown | Derived with the following derived variables: der_hotn_comp, der_MI_comp, der_card_isch_comp, der_AFib_comp, der_VF_comp, der_arry_oth_comp, der_CMY_comp, der_CHF_comp, der_PE_comp, der_DVT_comp, der_stroke_comp, der_thrombosis_NOS_comp Coded as 1 if any of these variables is 1; coded as 0 if all these variables are 0; coded as 99 if any of variables is 99 and der_CV_event_v2 is missing; otherwise, NA For all listed variable here: 0=No, 1=Yes, 99=Unknown |
| Derived pulmonary complication variable (see additional details) | der_pulm_event (der_any_Pulm is the variable name in R script) | 0=No; 1=Yes; 99=Unknown | Derived with the following derived variables: der_resp_failure_comp, der_pneumonitis_comp, der_pneumonia_comp, der_ARDS_comp, der_PE_comp, der_pleural_eff_comp, der_empyema_comp Coded as 1 if any of these variables is 1; coded as 0 if all these variables are 0; coded as 99 if any of variables is 99 and der_pulm_event is missing; otherwise, NA For all listed variable here: 0=No, 1=Yes, 99=Unknown |
| Derived gastrointestinal complication variable (see additional details) | der_GI_event (der_any_Gast is the variable name in R script) | 0=No; 1=Yes; 99=Unknown | Derived with the following derived variables: der_AHI_comp, der_ascites_comp, der_BO_comp, der_bowelPerf_comp, der_ileus_comp, der_peritonitis_comp Coded as 1 if any of these variables is 1; coded as 0 if all these variables are 0; coded as 99 if any of variables is 99 and der_GI_event is missing; otherwise, NA For all listed variable here: 0=No, 1=Yes, 99=Unknown |
| Acute kidney injury (checkbox only) | der_AKI_comp | 0=No; 1=Yes; 99=Unknown | |
| Multisystem organ failure | der_MOF_comp | 0=No; 1=Yes; 99=Unknown | |
| Any co-infection within ±2 weeks of COVID-19 dx | der_coinfection_any | 0=No; 1=Yes; 99=Unknown | |
| Sepsis | der_sepsis_comp | 0=No; 1=Yes; 99=Unknown | |
| Bleeding | der_bleeding_comp | 0=No; 1=Yes; 99=Unknown | |
| DIC (without modifier of definite/probable/possible) | der_DIC_comp | 0=No; 1=Yes; 99=Unknown | |
| Remdesivir as treatment for COVID-19 ever | der_rem | 0=No; 1=Yes; 99=Unknown | |
| Hydroxychloroquine as COVID-19 treatment ever | der_hcq | 0=No; 1=Yes; 99=Unknown | |
| Steroids as COVID-19 treatment ever | der_steroids_c19 | 0=No; 1=Yes; 99=Unknown | |
| COVID-19 treatments other than HCQ, steroids, remdesivir | der_other_tx_c19_v2 | 0=No; 1=Yes; 99=Unknown | |
| Indicates whether patient has ever had supplemental o2 | der_o2_ever | 0=No; 1=Yes; 99=Unknown |
Covariate description.
| Covariate description | Variable name | Covariate values | Additional details |
|---|---|---|---|
| Race/ethnicity including Asian | der_race_v2 | Hispanic; Non-Hispanic AAPI; Non-Hispanic Black; Non-Hispanic White; Other | |
| Age with imputation for categoricals | der_age_trunc | Years (continuous 18–89; patients noted to be greater than 89 are set to be age = 90) | |
| Insurance type | der_insurance | Medicaid alone; Medicare alone; Medicare/Medicaid ± other; Other government ± other; Private ± other; Uninsured; Unknown | |
| Derived variable for smoking status collapsing the current/former smoker variables | der_smoking2 | Never; Current or Former; Unknown | |
| Binary obesity (BMI ≥ 30 or checkbox checked) indicator | der_obesity | 0=No; 1=Yes; 99=Unknown | |
| Cardiovascular comorbidity (CAD, CHF, Afib, arrhythmia NOS, PVD, CVA, cardiac disease NOS) | der_card | 0=No; 1=Yes; 99=Unknown | |
| Derived variable indicating whether patient has pulmonary comorbidities | der_pulm | 0=No; 1=Yes; 99=Unknown | |
| Renal comorbidities | der_renal | 0=No; 1=Yes; 99=Unknown | |
| Derived variable indicating whether patient has diabetes mellitus | der_dm2 | 0=No; 1=Yes; 99=Unknown | |
| Performance status | der_ecogcat2 | ECOG 0, 1, or 2+ | |
| Breast biomarkers combined variable | der_breast_biomarkers | 1=ER + ; 2=ER + /HER2+; 3=HER2+; 4=triple negative; 99=Unknown | |
| Derived variable indicating cancer status (splits remission/NED by cancer timing) | der_cancer_status_v4 | 0 - Remission/NED, remote; 1 - Remission/NED, recent; 2 - Active, responding; 3 - Active, stable; 4 - Active, progressing; 99 - Unknown | |
| Timing of cancer treatment relative to COVID-19, collapsed | der_cancer_tx_timing_v2 | 0=more than 3 months; 1=0–4 weeks; 2=1–3 months (*); 88=never or after COVID-19 diagnosis; 99=unknown | |
| No cancer treatment in the 3 months prior to COVID-19 | der_cancertr_none | 0=No; 1=Yes; 99=Unknown | Derived with the following covariates: der_any_cyto, der_any_targeted, der_any_endo, der_any_immuno, der_any_local, der_any_other Coded as 1 if all these variables are 0; coded as 0 if any of these variables is 1; coded as 99 if any of these variables is 99; otherwise, NA |
| Any cytotoxic cancer treatment in the 3 months prior to COVID-19 | der_any_cyto | 0=No; 1=Yes; 99=Unknown | |
| Any targeted therapy in the 3 months prior to COVID-19 | der_any_targeted | 0=No; 1=Yes; 99=Unknown | |
| Any targeted therapy includes an anti-HER2 therapy in the 3 months prior to COVID-19 | der_her2_3 m | 0=No; 1=Yes | Derived with der_her2, der_any_targeted. Coded as 1 if der_any_targeted is 1 and der_her2 is 1 Coded as 0 if: a. der_any_targeted is 1 and der_her2 is 0 der_any_targeted is 1 Otherwise, NA der_her2: 0=No; 1=Yes |
| Any targeted therapy includes a CDK4/6 inhibitor therapy in the 3 months prior to COVID-19 | der_cdk46i_3 m | 0=No; 1=Yes | Derived with der_cdk46i, der_any_targeted. Coded as 1 if der_any_targeted is 1 and der_cdk46i is 1 Coded as 0 if: a. der_any_targeted is 1 and der_cdk46i is 0 der_any_targeted is 1 Otherwise, NA der_cdk46i: 0=No; 1=Yes |
| Any other targeted therapy (not anti-HER2/CDK4/6 inhibitor) in the 3 months prior to COVID-19 | der_other_3 m | 0=No; 1=Yes | Derived with der_targeted_not_her2_cdk46i, der_any_targeted. Coded as 1 if der_any_targeted is 1 and der_targeted_not_her2_cdk46i is 1 Coded as 0 if: a. der_any_targeted is 1 and der_targeted_not_her2_cdk46i is 0 der_any_targeted is 1 Otherwise, NA der_targeted_not_her2_cdk46i: 0=No; 1=Yes |
| Any endocrine therapy in the 3 months prior to COVID-19 | der_any_endo | 0=No; 1=Yes; 99=Unknown | |
| Any immunotherapy in the 3 months prior to COVID-19 | der_any_immuno | 0=No; 1=Yes; 99=Unknown | |
| Any local therapy (surgery or RT) within 3 months | der_any_local | 0=No; 1=Yes; 99=Unknown | |
| Any other cancer therapy in the 3 months prior to COVID-19 | der_any_other | 0=No; 1=Yes; 99=Unknown | |
| Region of patient residence with ex-US collapsed | der_region_v2 | Non-US; Other; Undesignated US; US Midwest; US Northeast; US South; US West | |
| Trimester and year of diagnosis, using the most recent side of the interval as anchor | der_tri_rt_dx | T1 2020; T2 2020; T3 2020; T1 2021 | |
| What type of area does the patient primarily reside in? | urban_rural1 | 1, Urban (city) | 2, Suburban (town, suburbs) | 3, Rural (country) | 88, Other | 99, Unknown | |
| The type of health care center providing the patient’s data | der_site_type | AMC = academic medical center; CP = community practice; TCC = tertiary care center | |
| Initial severity and course of illness | severity_of_covid_19_v21 | 1, Mild (no hospitalization required) | 2, Moderate (hospitalization indicated) | 3, Severe (ICU admission indicated) | 99, Unknown | |
| Derived treatment intent | der_tr_intent | Unknown Treatment; Not on Treatment; Palliative; Curative; Missing Unknown Treatment and Missing were collapsed for analysis | Derived with der_anytx and treatment_intent: Coded as ‘Unknown Treatment’ if der_anytx is NA or 99; Coded as ‘Not on Treatment’ if der_anytx is 0 Coded as ‘Palliative’ if der_anytx is 1 and treatment_intent is 2 Coded as ‘Curative’ if der_anytx is 1 and treatment_intent is 1 Otherwise, Missing der_anytx: 0=No; 1=Yes; 99=Unknown Treatment_intent: 1, Curative | 2, Palliative | 99, Unclear or unknown |
| Most recent line of cancer treatment, including systemic and non-systemic therapies | der_txline | Untreated in last 12 months; Curative NOS; First line; Non-curative NOS; Other; Second line or greater; Unknown | |
| Hematologic malignancy indicator | der_heme | 0=No; 1=Yes |
Other covariates used for analysis.
| Other covariate related to cohort selection for analysis | Variable name | Covariate values | Covariate description |
|---|---|---|---|
| Sex (recode other/prefer not to say gender -->missing) | der_sex | Male, Female | |
| Breast cancer | der_Breast | 0=No; 1=Yes | |
| Cancer type of second malignancy. If the patient has more than two malignancies, please select the second-most recently diagnosed cancer type. If unknown or unclear, please specify in the free text box below | cancer_type_21 | ‘’ indicates no second malignancy | |
| Region of patient residence with US and ex-US collapsed | der_region_v3 | Non-US; Other; US |
New covariates added (2-5-22).
| New covariate | Variable name | Covariate values | Covariate description |
|---|---|---|---|
| MBC vs non-MBC | der_metastatic | 0=No; 1=Yes; 99=Unknown | Metastatic cancer status (only applicable to solid tumors/lymphoma) |
| MBC site of metastasis | der_met_bone | 0=No; 1=Yes; 99=Unknown | Metastatic to bone |
| MBC site of metastasis | der_met_liver | 0=No; 1=Yes; 99=Unknown | Metastatic to liver |
| MBC site of metastasis | der_met_lung_v2 | 0=No; 1=Yes; 99=Unknown | Metastatic to lung |
Unadjusted rates of outcomes after COVID-19 diagnosis by cancer status.
| NED >5years | NED <5years | Active and responding | Active andstable | Active and progressing | Missing/ unknown | Total | |
|---|---|---|---|---|---|---|---|
| n* (%) | n* (%) | n* (%) | n* (%) | n* (%) | n* (%) | n* (%) | |
| Outcomes | |||||||
| Total all-cause mortality† | 40 (11) | 12 (3) | 12 (7) | 11 (7) | 37 (38) | 11 (9) | 123 (9) |
| 30-day all-cause mortality ‡ | 29 (8) | 10 (2) | 10 (6) | 4 (2) | 27 (28) | 9 (7) | 89 (6) |
| Received mechanical ventilation† | 20 (5) | 13 (3) | 9 (5) | 7 (4) | 12 (12) | 8 (7) | 69 (5) |
| Admitted to an intensive care unit† | 35 (10) | 25 (6) | 13 (8) | 8 (5) | 18 (19) | 12 (10) | 111 (8) |
| Admitted to the hospital† | 163 (43) | 129 (29) | 54 (31) | 57 (34) | 70 (72) | 39 (32) | 512 (37) |
-
*
N is based on non-missing data.
-
†
Included in primary ordinal COVID-19 severity outcome.
-
‡
Secondary outcome.
Baseline characteristics of female patients with MBC and COVID-19.
| MBC | |
|---|---|
| (N=233) | |
| Age, years† | |
| Median [IQR] | 58.0 [49.8, 68.3] |
| Race/ethnicity | |
| Non-Hispanic White | 107 (46%) |
| Non-Hispanic Black | 56 (24%) |
| Hispanic | 50 (21%) |
| Non-Hispanic AAPI | 10 (4%) |
| Other | 10 (4%) |
| Smoking status | |
| Never | 162 (70%) |
| Current or former | 66 (28%) |
| Missing/unknown | 5 (2%) |
| Obesity | |
| No | 139 (60%) |
| Yes | 93 (40%) |
| Comorbidities‡ | |
| Cardiovascular | 42 (18%) |
| Pulmonary | 37 (16%) |
| Renal disease | 16 (7%) |
| Diabetes mellitus | 52 (22%) |
| Missing/unknown | 3 (1%) |
| ECOG performance status | |
| 0 | 63 (27%) |
| 1 | 84 (36%) |
| 2+ | 42 (18%) |
| Unknown | 44 (19%) |
| Missing | 0 (0%) |
| Receptor status | |
| HR+/HER2- | 98 (42%) |
| HR+/HER2+ | 53 (23%) |
| HR-/HER2+ | 26 (11%) |
| Triple negative | 33 (14%) |
| Missing/unknown | 23 (10%) |
| Cancer status | |
| Active and responding | 55 (24%) |
| Active and stable | 78 (33%) |
| Active and progressing | 74 (32%) |
| Unknown | 25 (11%) |
| Missing | 0 (0%) |
| Metastatic sites (MBC) | |
| Lung | 65 (28%) |
| Bone | 135 (58%) |
| Liver | 61 (26%) |
| Missing/unknown | 19 (8%) |
| Timing of anti-cancer therapy | |
| Never/after COVID-19 | X* |
| 0–4 weeks | 189 (81%) |
| 1–3 months | 14 (6%) |
| >3 months | 19 (8%) |
| Missing/unknown | 11 (5%)* |
| Modality of active anti-cancer therapy‡, § | |
| None | 24 (10%) |
| Cytotoxic chemotherapy | 114 (49%) |
| Targeted therapy | 115 (49%) |
| Endocrine therapy | 98 (42%) |
| Immunotherapy | 17 (7%) |
| Local (surgery/radiation) | 27 (12%) |
| Other | 6 (3%) |
| Missing/unknown | 6 (3%) |
| Region | |
| Northeast | 97 (42%) |
| Midwest | 44 (19%) |
| South | 34 (15%) |
| West | 56 (24%) |
| Undesignated | 2 (1%) |
| Period of COVID-19 diagnosis | |
| Jan-Apr 2020 | 33 (14%) |
| May-Aug 2020 | 101 (43%) |
| Sept-Dec 2020 | 52 (22%) |
| Jan-Aug 2021 | 45 (19%) |
| Missing/unknown | 2 (1%) |
| Area of patient residence | |
| Urban | 103 (44%) |
| Suburban | 80 (34%) |
| Rural | 12 (5%) |
| Missing/unknown | 38 (16%) |
| Treatment center characteristics | |
| Academic medical center | 43 (18%) |
| Community practice | 63 (27%) |
| Tertiary care center | 127 (55%) |
| Missing/unknown | 0 (0%) |
| Severity of COVID-19 | |
| Mild | 126 (54%) |
| Moderate | 93 (40%) |
| Severe | 13 (6%) |
| Missing/unknown | 1 (<1%) |
-
*
Cells combined to mask N<5 according to CCC19 low count policy.
-
†
Age was truncated at 90 years.
-
‡
Percentages could sum to >100% because categories are not mutually exclusive.
-
§
Within 3 months of COVID-19 diagnosis.
Unadjusted rates of outcomes after COVID-19 diagnosis in female patients with MBC.
| n** (%) | |
|---|---|
| Outcomes | |
| Total all-cause mortality* | 45 (19) |
| 30-day all-cause mortality† | 28 (12) |
| Received mechanical ventilation* | 20 (9) |
| Admitted to an intensive care unit* | 29 (12) |
| Admitted to the hospital* | 124 (53) |
| Clinical complications | |
| Any cardiovascular complication‡ | 48 (21) |
| Any pulmonary complication§ | 86 (37) |
| Any gastrointestinal complication¶ | 13 (6) |
| Acute kidney injury | 32 (14) |
| Multisystem organ failure | 12 (5) |
| Superimposed infection | 32 (14) |
| Sepsis | 28 (12) |
| Any bleeding | 8 (3) |
| Interventions | |
| Remdesivir | 35 (15) |
| Hydroxychloroquine | 25 (11) |
| Corticosteroids | 65 (29) |
| Covid Other | 45 (20) |
| Supplemental oxygen | 84 (37) |
-
*
Included in primary ordinal COVID-19 severity outcome.
-
†
Secondary outcome.
-
‡
Cardiovascular complication includes hypotension, myocardial infarction, other cardiac ischemia, atrial fibrillation, ventricular fibrillation, other cardiac arrhythmia, cardiomyopathy, congestive heart failure, pulmonary embolism (PE), deep vein thrombosis (DVT), stroke, thrombosis NOS complication.
-
§
Pulmonary complication includes respiratory failure, pneumonitis, pneumonia, acute respiratory distress syndrome (ARDS), PE, pleural effusion, empyema.
-
¶
Gastrointestinal complication includes acute hepatic injury, ascites, bowel obstruction, bowel perforation, ileus, peritonitis.
-
**
N is based on non-missing data.
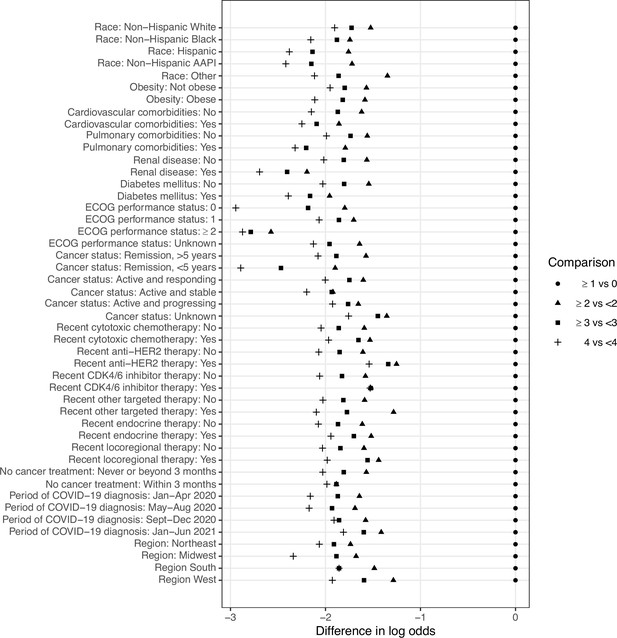
Adjusted associations of race factors with COVID-19 severity outcome.
Baseline characteristics of male patients with breast cancer and COVID-19.
| Total | 25 (100%) |
| Age, years† | |
| Median [IQR] | 67.0 [60–75] |
| Race/ethnicity | |
| NHW | 13 (52%) |
| Black | 8 (32%) |
| Hispanic | <5 (<20%) |
| AAPI | 0 (0%) |
| Other | <5 (<20%) |
| Smoking status | |
| Never | 18 (72%) |
| Current or former | 7 (28%) |
| Obesity | |
| No | 12 (48%) |
| Yes | 13 (52%) |
| Comorbidities ‡ | |
| Cardiovascular | 6 (24%) |
| Pulmonary | 5 (20%) |
| Renal disease | <5 (<20%) |
| Diabetes mellitus | 11 (44%) |
| ECOG performance status | |
| 0 | 5 (20%) |
| 1 | 10 (40%) |
| 2+ | X* |
| Unknown | 10 (40%)* |
| Receptor status | |
| HR+/HER2- | 18 (72%) |
| HR+/HER2+ | 5 (20%) |
| HR+/HER2+ | X* |
| Triple negative | 0 (0%) |
| Missing/unknown | 2 (8%)* |
| Cancer status | |
| Remission or NED, >5 years | <5 (<20%) |
| Remission or NED, <5 years | 6 (24%) |
| Active and responding | <5 (<20%) |
| Active and stable | <5 (<20%) |
| Active and progressing | 5 (20%) |
| Unknown | 3 (12%) |
| Timing of anti-cancer therapy | |
| Never/after COVID-19 | <5 (<20%) |
| 0–4 weeks | 17 (68%) |
| 1–3 months | 0 (0%) |
| >3 months | <5 (<20%) |
| Missing/unknown | 1 (4%) |
| Modality of active anti-cancer therapy‡, § | |
| None | 7 (28%) |
| Chemotherapy | 6 (24%) |
| Targeted therapy | 6 (24%) |
| Endocrine therapy | 10 (40%) |
| Immunotherapy | 0 (0%) |
| Local (surgery/radiation) | <5 (<20%) |
| Other | 0 (0%) |
| Missing/unknown | 1 (4%) |
| Region | |
| Northeast | 11 (44%) |
| Midwest | <5 (<20%) |
| South | <5 (<20%) |
| West | 7 (28%) |
| Undesignated | 0 (0%) |
| Period of COVID-19 diagnosis | |
| Jan-Apr 2020 | 10 (40%) |
| May-Aug 2020 | 9 (36%) |
| Sept-Dec 2020 | 5 (20%) |
| Area of patient residence | |
| Urban | 9 (36%) |
| Suburban | 8 (32%) |
| Rural | 0 (0%) |
| Missing/unknown | 8 (32%) |
| Severity of COVID19 | |
| Mild | 11 (44%) |
| Moderate/severe | 14 (56%) |
-
Variable categories with one to five cases are masked by replacing with N<5 according to CCC19 policy.
-
*
Cells combined to mask N<5 according to CCC19 low count policy.
-
†
Age was truncated at 90 years.
-
‡
Percentages could sum to >100% because categories are not mutually exclusive.
-
§
Within 3 months of COVID-19 diagnosis.
Unadjusted rates of outcomes after COVID-19 diagnosis among male patients with BC.
| Outcomes | |
|---|---|
| Total all-cause mortality | 5 (20) |
| 30-day all-cause mortality | 5 (20) |
| Received mechanical ventilation | <5 (<20%) |
| Admitted to an intensive care unit | <5 (<20%) |
| Admitted to the hospital | 15 (60) |
| Clinical complications | |
| Any cardiovascular complication* | <5 (<20%) |
| Any pulmonary complication† | 12 (48) |
| Any gastrointestinal complication‡ | 0 (0%) |
| Acute kidney injury | <5 (<20%) |
| Multisystem organ failure | <5 (<20%) |
| Superimposed infection | <5 (<20%) |
| Sepsis | <5 (<20%) |
| Any bleeding | <5 (<20%) |
| Interventions | |
| Remdesivir | <5 (<20%) |
| Hydroxychloroquine | 7 (28) |
| Corticosteroids | <5 (<20%) |
| Other | 9 (36) |
| Supplemental oxygen | 12 (48) |
-
Variable categories with one to five cases are masked by replacing with N<5 according to CCC19 policy
-
*
Cardiovascular complication includes hypotension, myocardial infarction, other cardiac ischemia, atrial fibrillation, ventricular fibrillation, other cardiac arrhythmia, cardiomyopathy, congestive heart failure, pulmonary embolism (PE), deep vein thrombosis (DVT), stroke, thrombosis NOS complication.
-
†
Pulmonary complication includes respiratory failure, pneumonitis, pneumonia, acute respiratory distress syndrome (ARDS), PE, pleural effusion, empyema.
-
‡
Gastrointestinal complication includes acute hepatic injury, ascites, bowel obstruction, bowel perforation, ileus, peritonitis.