Drug specificity and affinity are encoded in the probability of cryptic pocket opening in myosin motor domains
Figures
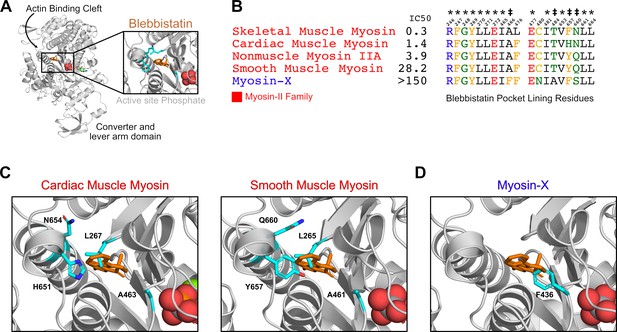
Blebbistatin’s cryptic binding pocket is closed in blebbistatin-free experimental myosin structures and the sequence of surrounding residues is highly conserved across myosin isoforms with widely varying IC50s.
(A) The motor domain of a D.d. myosin-II molecule bound to blebbistatin. The inset depicts blebbistatin’s cryptic binding pocket (PDB: 1YV324). Blebbistatin is shown in orange sticks while the active site phosphate is shown in spheres. Select residues (same as those shown in C) are shown in cyan sticks. (B) Alignment of blebbistatin contact residues (within 5 Å of blebbistatin in 1YV3) reveals that 16 of 19 residues are identical among myosin-IIs despite almost two orders of magnitude difference in blebbistatin IC50. We also include an unconventional myosin-X to highlight an important sequence difference at residue 466 (A vs. F) that separates blebbistatin-sensitive (IC50 <150 μM) and blebbistatin-insensitive isoforms (IC50 >150 μM). A star indicates a residue is conserved among all myosin isoforms shown. A double cross is used to indicate sequence differences previously suggested to play an important role in determining blebbistatin specificity (Allingham et al., 2005). (C) Crystal structures of β-cardiac (PDB: 5N6A Planelles-Herrero et al., 2017) and smooth muscle myosin (1BR2 Dominguez et al., 1998) do not suggest an obvious mechanism for differences in blebbistatin potency between these isoforms. In both structures, the cryptic blebbistatin-binding pocket is closed, and an aligned blebbistatin molecule (orange) clashes with a leucine residue (cyan). The two residues that differ between these isoforms (cyan histidine and asparagine in cardiac; cyan tyrosine and glutamine in smooth) do not form specific interactions with blebbistatin (e.g. hydrogen bonds). (D) In a blebbistatin-free myosin-X structure (PDB: 5I0H Ropars et al., 2016), F436 (cyan) forms a large steric impediment to blebbistatin binding (aligned molecule shown in orange) that is not present in myosin-II isoforms.
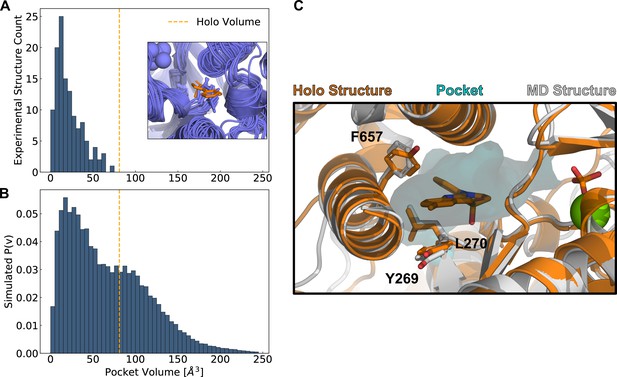
Simulations reveal opening of blebbistatin’s cryptic pocket.
(A) The distribution of pocket volumes from experimental crystal structures queried from the Protein Data Bank shows that the blebbistatin pocket is cryptic. The inset is a random selection of 15 structures from the accompanying distribution with an overlaid blebbistatin molecule in orange. All experimentally determined myosin structures display steric clash with a blebbistatin molecule aligned based on its contact residues in a blebbistatin-bound, or holo, structure (PDB: 1YV3). (B) Blebbistatin pocket volumes in simulations of fast skeletal myosin IIA reveal substantial pocket opening. The blebbistatin pocket volume from a ligand-bound crystal structure (PDB: 1YV3) is delineated by an orange vertical line in both panels. Simulated P(v) corresponds to the probability of adopting a given volume for each bin in the histogram. (C) MD simulations explore open holo-like states. Structure of an open conformation of the blebbistatin binding pocket from MD simulations reveals good structural alignment with the holo crystal structure (0.55 Å root mean square deviation of contact residue backbone heavy atom and Cβ positions). Blebbistatin is shown in orange with the pocket from the MD structure shown as a cyan contour. Selected residues in the blebbistatin pocket (Y269, L270, and F657) have the same backbone and sidechain positions as in the holo crystal structure. Note that reported pocket volumes are smaller than the space available to ligands because of an algorithm choice made to avoid erroneous detection of small pockets (see Materials and methods for details).
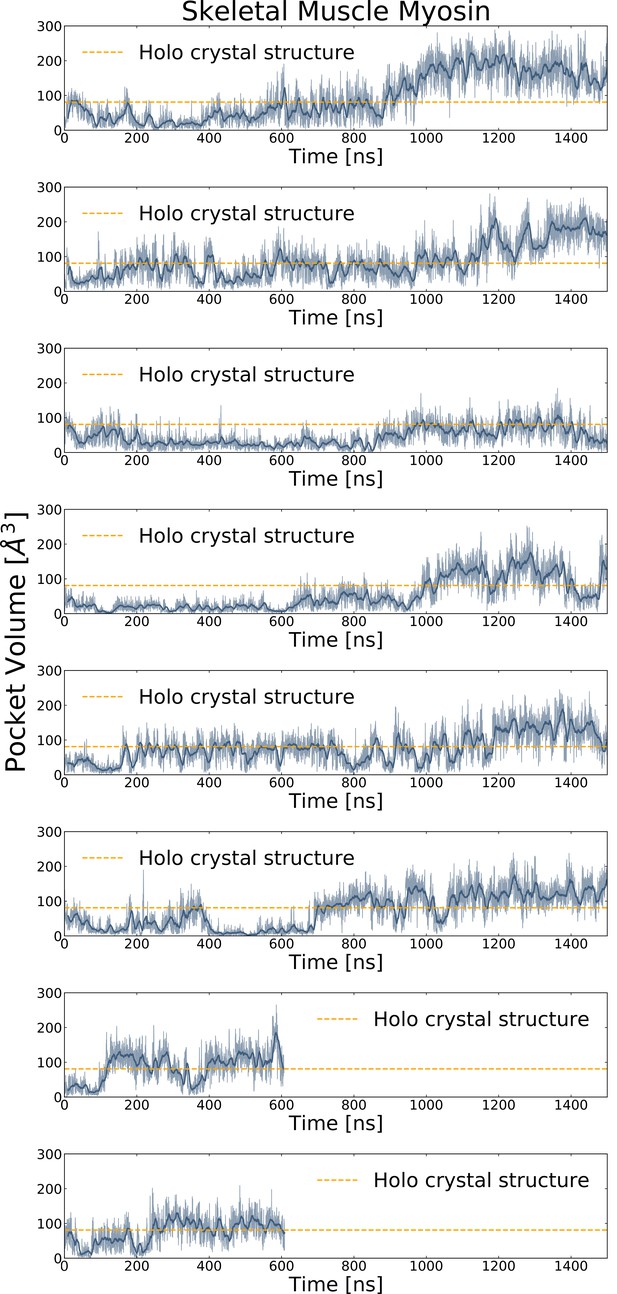
Trajectory traces for long simulations of skeletal muscle myosin reveal opening in all long MD trajectories.
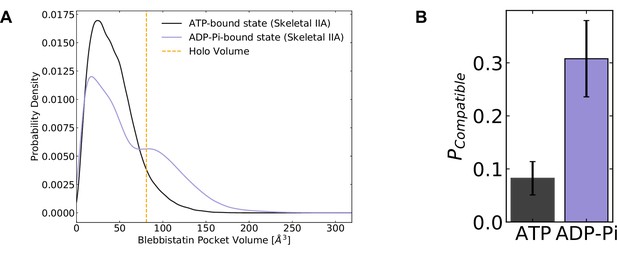
Blebbistatin pocket opening preferentially occurs in the ADP*Pi state.
(A) Distributions of skeletal muscle myosin IIA blebbistatin pocket volumes in the ATP-bound state and ADP*Pi-bound state demonstrate that the blebbistatin pocket is more likely to open in the ADP*Pi-bound state, consistent with biochemical experiments which predict tighter binding between blebbistatin and myosin when myosin is bound to ADP*Pi. (B) The probability of adopting compatible structures (i.e. structures with pocket volumes equal to or greater than the blebbistatin-bound crystal structure) is higher when myosin is bound to ADP and phosphate. Error bars represent estimate of standard error of the mean from 250 trials of bootstrapping where trajectories were drawn with replacement from the entire dataset (see Materials and methods). Note that reported pocket volumes are smaller than the space available to ligands because of an algorithm choice made to avoid erroneous detection of small pockets (see Materials and methods for details).
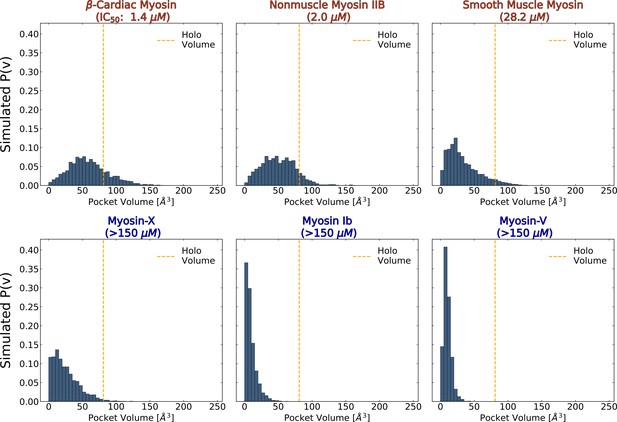
The probability of adopting open pocket conformations is greater among blebbistatin-sensitive isoforms (top row) than insensitive isoforms (bottom row).
MSM-weighted distributions of blebbistatin pocket volumes in simulations of nucleotide-free isolated myosin motor domains show that myosin-IIs (top row) are more likely to exceed the blebbistatin pocket volume of a holo crystal structure (PDB: 1YV3, orange line) than non-class II myosins. Among myosin-IIs, those with lower IC50s (Limouze et al., 2004; Eddinger et al., 2007; Wang et al., 2008; Zhang et al., 2017; Várkuti et al., 2016; Radnai, 2021) have more right-shifted pocket volume distributions. The overall opening probabilities between these isoforms can be visualized in Figure 4—figure supplement 1. Note that reported pocket volumes are smaller than the space available to ligands because of an algorithm choice made to avoid erroneous detection of small pockets (see Materials and methods for details).
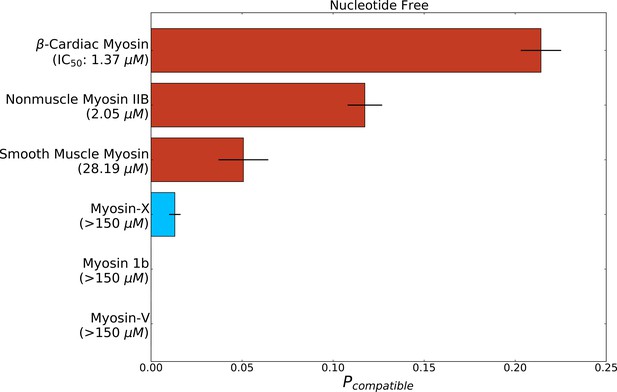
The probability of adopting open pocket conformations is greater among blebbistatin-sensitive isoforms (red bars) than insensitive isoforms (blue bars).
Structural states were considered compatible if the pocket volume at the blebbistatin binding site matched or exceeded that of a holo crystal structure (PDB: 1YV3). Error bars represent the standard error of the mean from 250 trials of bootstrapping where trajectories were drawn with replacement from the entire dataset.
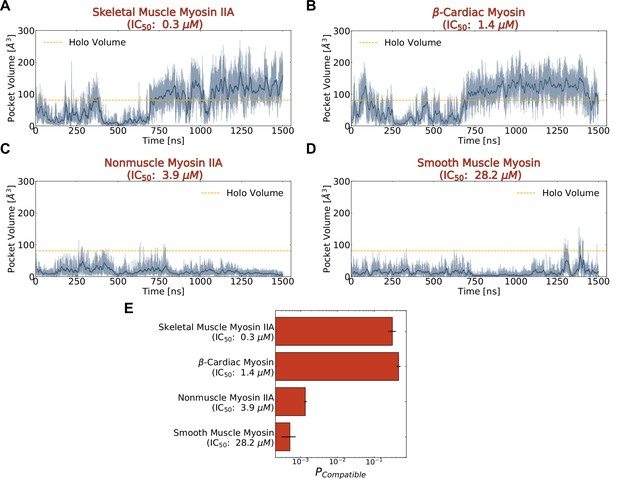
Blebbistatin cryptic pocket opening is more likely in simulations of highly sensitive myosin-II isoforms than in simulations of less sensitive myosin-IIs.
(A-D) Representative pocket volume trajectory traces for several myosin-II isoforms show that pocket opening occurs with greater frequency and that the pocket stays open for longer in those isoforms more potently inhibited by blebbistatin (top row). The dotted orange line delineates the blebbistatin pocket volume in a holo crystal structure (PDB ID: 1YV3). Transparent blue lines indicate raw data while the opaque blue lines are a 10 ns rolling average. (E) Blebbistatin pocket opening is highly probable (>0.25) in skeletal muscle myosin II and β-cardiac myosin but highly unlikely (<0.01) for nonmuscle myosin IIA and smooth muscle myosin. A conformation was considered compatible if its blebbistatin pocket volume exceeded the pocket volume of a holo crystal structure (PDB ID: 1YV3). Conformations were weighted by their equilibrium probability in Markov State Models of the blebbistatin pocket. Error bars show bootstrapped estimate of standard error of the mean from 250 trials. Note that reported pocket volumes are smaller than the space available to ligands because of an algorithm choice made to avoid erroneous detection of small pockets (see Materials and methods for details).
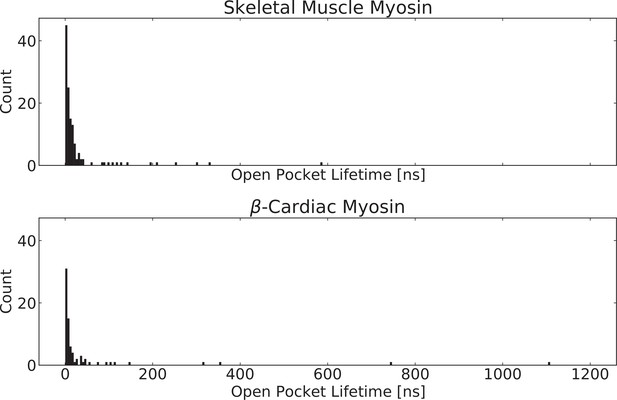
The blebbistatin pocket stays open for prolonged periods (>200 ns) of simulation time in both skeletal and β-cardiac myosin.
We determined the open pocket lifetime from trajectory traces of pocket volume. Specifically, we took a 10 ns window average and determined how long the pocket volume exceeded that of the holo structure (PDB: 1YV3) for each opening event (i.e. trajectory time from opening to closing). For smooth muscle myosin and nonmuscle myosin 2 A, a 10 ns rolling window average never exceeded the holo volume, although individual structures do.
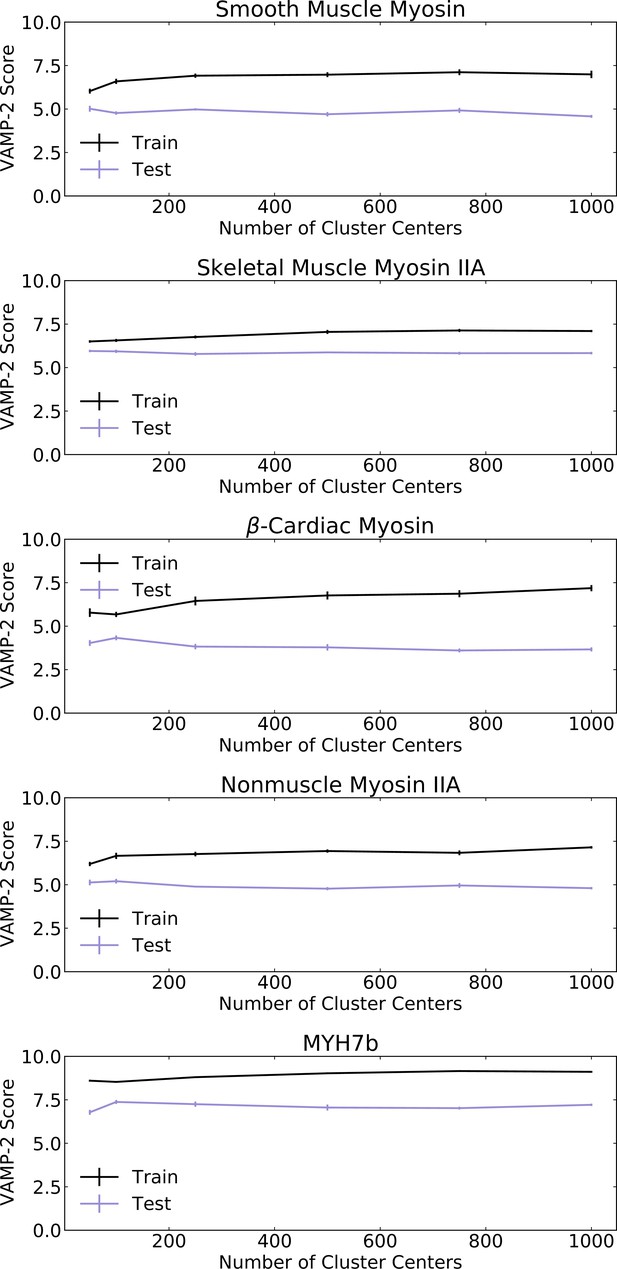
VAMP-2 (Variational approach for Markov processes) scores for MSMs constructed with varying numbers of cluster centers were computed on a validation set of trajectories to select an appropriate number of cluster centers for each MSM.
Error bars show standard error of mean VAMP-2 score.
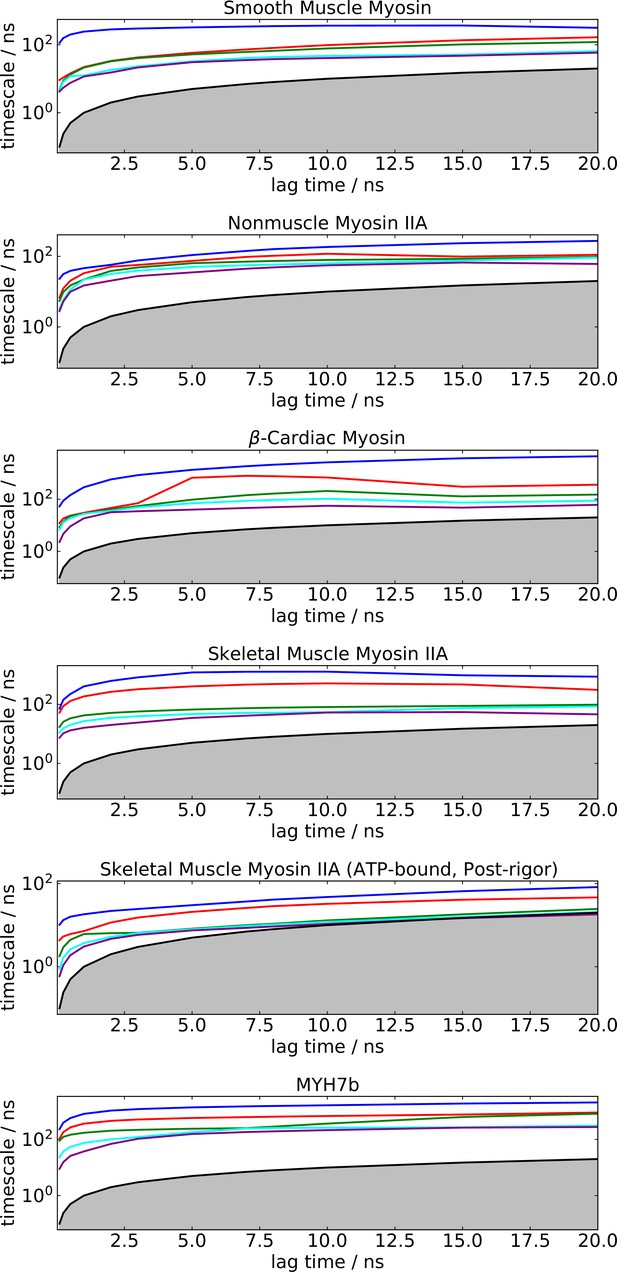
implied timescales for Markov State Models of the blebbistatin pocket across multiple myosin isoforms show convergence on a logarithmic scale.
The gray area indicates the region where timescales become equal to or smaller than the lag time and can no longer be resolved.
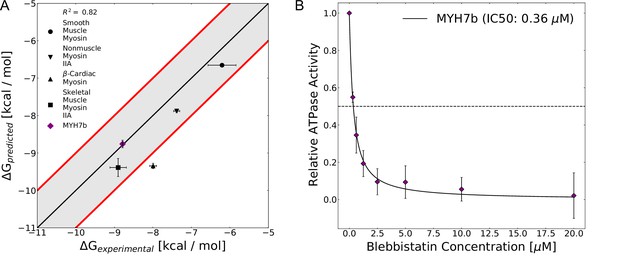
The computed free energy of binding for blebbistatin from MSM-docking accurately predicts binding free energies for existing experimental data and for a myosin isoform whose blebbistatin sensitivity was not known.
(A) Predictions from MSM-docking are highly correlated to experimental values (R2=0.82) and most predictions are within 1 kcal/mol of experimental values. Error bars for predicted free energies of binding represent bootstrapped estimate of standard error of the mean from 250 trials. Error bars for experimental values show the standard error of the IC50 or Ki converted to a binding free energy. (B) An NADH-linked ATPase assay indicates that MYH7b is highly sensitive to blebbistatin inhibition (IC50: 0.36 μM), consistent with the prediction from MSM-docking. Data show the mean ATPase activity ± standard deviation across 5 experimental replicates (2 biological replicates, each with two or three technical replicates).
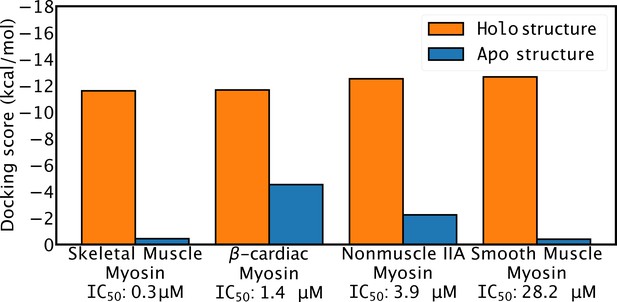
Docking scores to homology models of apo and holo structures do not correlate with blebbistatin potency.
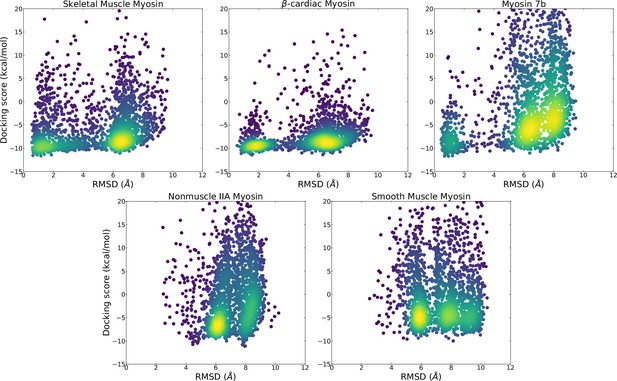
Comparison of distribution of docking scores and ligand heavy atom root mean square deviation (RMSD) from blebbistatin’s pose in a holo crystal structure (PDB: 1YV3) show that skeletal and β-cardiac myosin are more likely to adopt structures where blebbistatin can be docked in its holo orientation and obtain a favorable docking score.
Points are colored by density with bright colors indicate areas of high density.
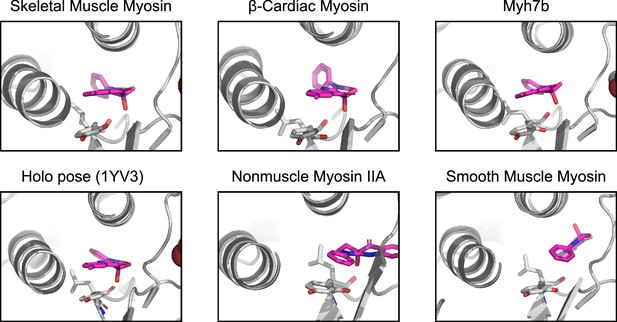
Highest scoring poses for each of the myosin isoforms reveals that the best pose for skeletal muscle myosin, β-cardiac myosin, and Myh7b closely matches the pose seen in holo crystal structures (ligand heavy atom RMSD 1.2 Å, 1.5 Å, and 0.9 Å to holo PDB 1YV3 for skeletal muscle myosin, β-cardiac myosin, and Myh7b, respectively).
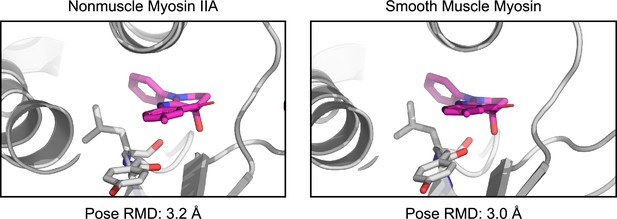
Docking to nonmuscle myosin IIA and smooth muscle myosin produces low RMSD poses (3.2 Å and 3.0 Å blebbistatin heavy atom RMSD from holo 1YV3 structure) with reasonably high scores (–6.3 kcal/mol in both cases).
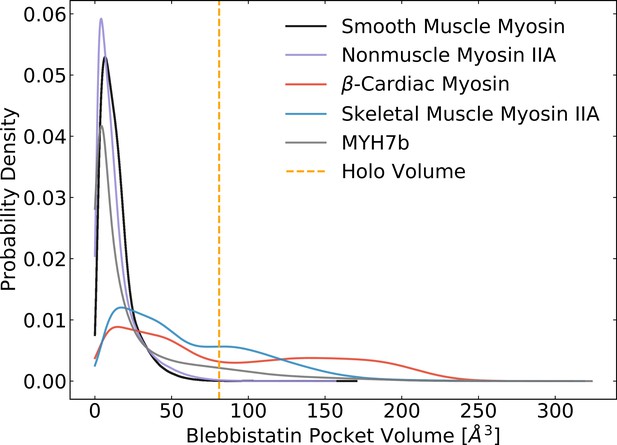
MSM-weighted pocket volumes for myosin-II isoforms in the ADP*Pi state reveal that blebbistatin pocket opening commonly occurs in skeletal muscle myosin, β-cardiac myosin, and Myh7b.
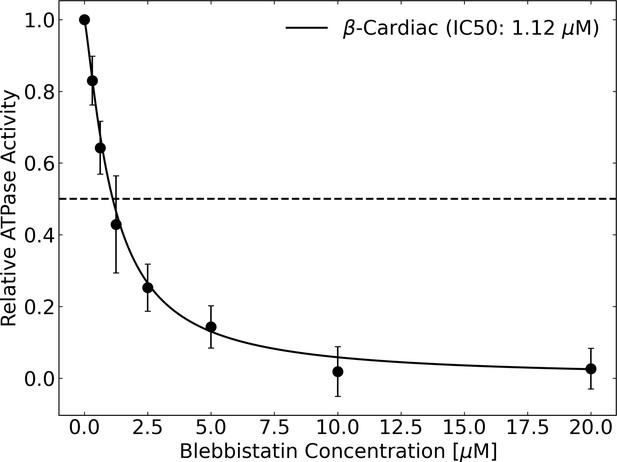
Blebbistatin inhibits the actin-activated ATPase activity of β-cardiac myosin with an IC50 of 1.12 μM.
ATPase was measured using an NADH-linked assay. Error bars represent standard deviation across four trials (two biological replicates with two technical replicates each).
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Gene (H. sapiens) | β-MyHC S1 | UNIPROT: P12883 | amino acids 1–842 | |
| Gene (H. sapiens) | MYH7b S1 | PMID:36334627 | amino acids 1–850 | |
| Cell line (M. musculus) | C2C12 cells | PMID:20080549 ATCC | ||
| Chemical compound, drug | Blebbistatin | Selleckchem | S7099 | |
| Software, algorithm | GROMACS | https://doi.org/10.1016/j.softx.2015.06.001 | Version 2021.1 |
Homology models prepared for this study.
| Isoform | Gene | UniprotID | Templatestructure | Structural state / Nucleotide state |
|---|---|---|---|---|
| Fast Skeletal | MYH2 | Q9UKX2 | 5N6A | PPS (ADP*Pi) |
| β-Cardiac | MYH7 | P12883 | 5N6A | PPS (ADP*Pi) |
| Nonmuscle IIA | MYH9 | P35579 | 5I4E | PPS (ADP*Pi) |
| Smooth | MYH11 | P35749 | 1BR2 | PPS (ADP*Pi) |
| MYH7b | MYH7B | A7E2Y1 | 5N6A | PPS (ADP*Pi) |
| Fast Skeletal | MYH2 | P12883 | 6FSA | PR (ATP) |
-
PPS indicates prepowerstroke while PR indicates post rigor.
Sequence similarity between sequence used for homology modeling and template structures.
| Gene | Templatestructure | Sequence identity | Sequence similarity |
|---|---|---|---|
| MYH2 | 5N6A | 80% | 88% |
| MYH7 | 5N6A | 96% | 98% |
| MYH9 | 5I4E | 78% | 90% |
| MYH11 | 1BR2 | 94% | 96% |
| MYH7B | 5N6A | 69% | 85% |
| MYH2 | 6FSA | 80% | 88% |
Simulation length and Markov State Model hyperparameters for myosin isoforms.
| System | Structural state | Number of cluster centers | Lag time (ns) | Total simulation time (µs) | Median trajectory length(ns) | Maximum trajectory length(ns) |
|---|---|---|---|---|---|---|
| Skeletal Muscle Myosin | ADP*Pi | 50 | 5 | 89.0 | 21.2 | 1500 |
| β-Cardiac Muscle Myosin | ADP*Pi | 100 | 5 | 90.1 | 21.0 | 1500 |
| Nonmuscle Myosin IIA | ADP*Pi | 100 | 8 | 86.7 | 20.0 | 1500 |
| Smooth Muscle Myosin | ADP*Pi | 50 | 5 | 87.0 | 20.0 | 1500 |
| Skeletal Muscle Myosin | ATP | 100 | 5 | 2.7 | 910.0 | 925 |
| Myosin 7b | ADP*Pi | 100 | 5 | 100.8 | 375.0 | 1500 |
Additional files
-
MDAR checklist
- https://cdn.elifesciences.org/articles/83602/elife-83602-mdarchecklist1-v2.docx
-
Supplementary file 1
The following supplementary data tables are available: IC50 values for different myosin isoforms; Percent identity in motor domain sequence between myosin-II isoforms in this study; Structural similarity between myosin-II prepowerstroke state crystal structures as assessed by C- α root mean square deviation.
- https://cdn.elifesciences.org/articles/83602/elife-83602-supp1-v2.docx