Decoupled neoantigen cross-presentation by dendritic cells limits anti-tumor immunity against tumors with heterogeneous neoantigen expression
Figures
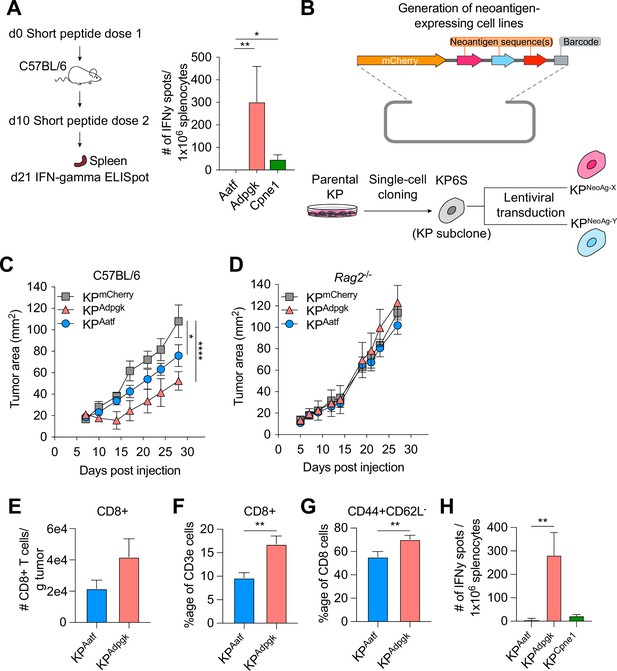
KP6S cell line engineered to express natural neoantigens (NeoAgs) elicit variable anti-tumor immune responses.
(A) Mice were vaccinated with short peptides with cyclic-di-GMP as adjuvant. 10 µg of peptide was delivered subcutaneously (s.c.) at the base of the tail along with 25 µg of cyclic-di-GMP. An identical dose was delivered s.c. 10 days following the first dose and spleens were collected at day 21 for IFNγ ELISpot. Quantification of IFNγ-producing cells after restimulation from two independent experiments shown as mean ± SEM (n = 3 per group per experiment). (B) Schematic of the lentiviral construct used to transduce the KP6S subclone. (C, D) Mice were injected s.c. with 1 × 106 tumor cells in (B) WT mice or (C) Rag2-/- mice. Representative data from one of two individual experiments are shown (n = 3 or 4 per group per experiment). Quantification of (E) absolute numbers of CD8+ TIL per gram tumor from six independent experiments (pooled n = 17 per group), (F) proportion of CD8+ TIL at day 9 or 10 after tumor implantation from eight independent experiments (pooled n = 23 per group), (G) proportion of CD44+CD62L- Teffector from eight independent experiments (pooled n = 23 per group), (H) IFNγ-producing cells restimulated 9 or 10 d after tumor implantation using ELISpot from two independent experiments (pooled n = 5 per group). *p<0.05, **p<0.01, ****p<0.0001; one-way ANOVA (Kruskal–Wallis) test in (A), two-way ANOVA (Tukey) in (C, D), Mann–Whitney U in (E–H). Data are shown as mean ± SEM.
-
Figure 1—source data 1
Raw data for Figure 1.
- https://cdn.elifesciences.org/articles/85263/elife-85263-fig1-data1-v3.xlsx
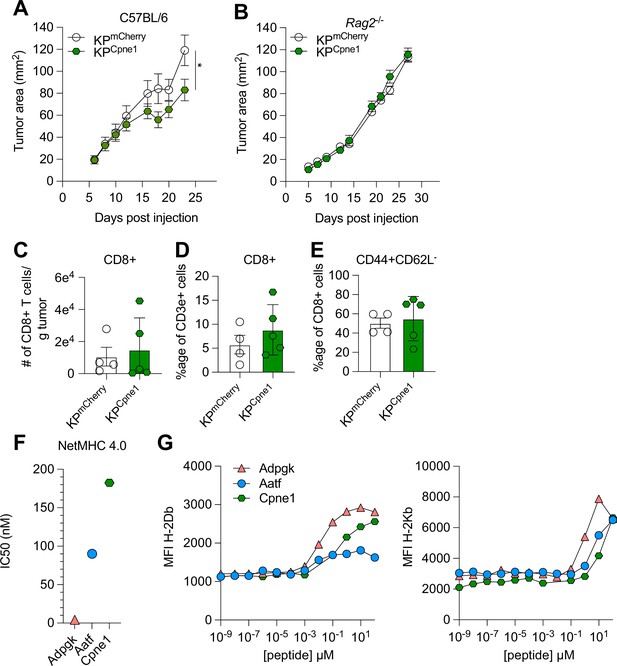
Characterization of the immunogenicity of an array of natural neoantigens (NeoAgs).
(A, B) Mice were injected subcutaneously with 1 × 106 tumor cells in (A) WT mice or (B) Rag2-/- mice. Representative data from one of two individual experiments are shown (n = 5 per group per experiment) in (A) and one individual experiment (n = 4 per group) in (B). Quantification of (C) absolute numbers of CD8+ TIL per gram tumor from two independent experiments (pooled n = 4 or 5 per group), (D) proportion of CD8+ TIL at day 9 or 10 after tumor implantation from two independent experiments (pooled n = 4 or 5 per group), (E) proportion of CD44+CD62L- Teffector from two independent experiments (pooled n = 4 or 5 per group). (F) Predicted binding affinity values measured as IC50 values (nM) from NetMHCpan-4.0 server. (G) RMA-S cells were incubated with a titration of individual peptides and then stained with antibodies to discern preferential binding to H-2Db (left) or H-2Kb (right). *p<0.05; two-way ANOVA (Bonferroni) in (A, B), Mann×Whitney U in (C–E). Data are shown as mean ± SEM.
-
Figure 1—figure supplement 1—source data 1
Raw data for Figure 1—figure supplement 1.
- https://cdn.elifesciences.org/articles/85263/elife-85263-fig1-figsupp1-data1-v3.xlsx
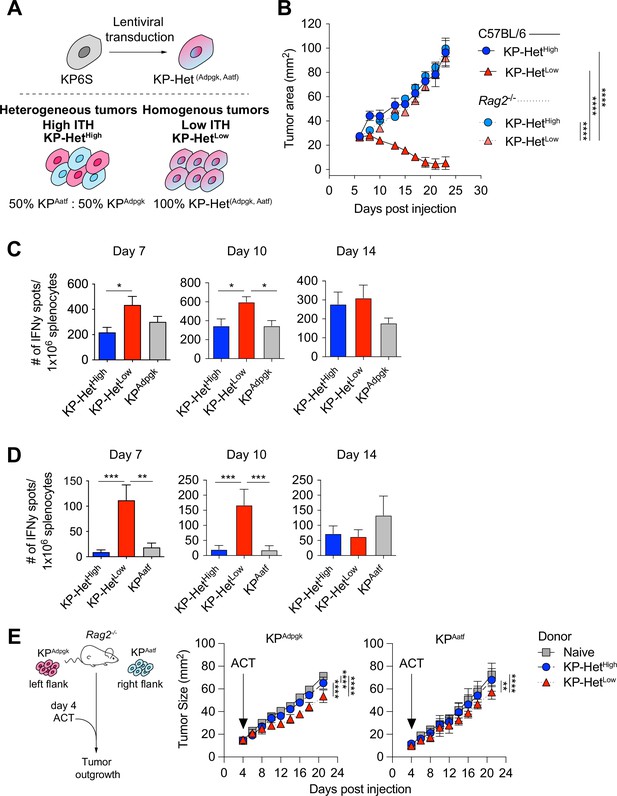
Tumors expressing a pair of neoantigens (NeoAgs) homogeneously have increased immunogenicity.
(A) Schematic of the generation of tumors used in (B). (B) Tumor growth of KP-HetHigh and KP-HetLow in WT and Rag2-/- mice. Representative data from one of two individual experiments are shown (n = 3 per group per experiment). (C, D) Splenocytes from tumor-bearing mice were used in an IFNγ ELISpot to determine the frequency of NeoAg-specific T cells in the periphery at days 7, 10, and 14 after tumor implantation. Quantification of the (C) Adpgk-specific response and (D) Aatf-specific response. Pooled data from five independent experiments for day 7 for single antigen tumors and six independent experiments for all other groups (n = 3–4 per group per experiment), four independent experiments for day 10 for single antigen tumors and five independent experiments for all other groups (n = 3 per group per experiment) and three independent experiments for day 14 (n = 3 per group per experiment) in (C, D). (E) Schematic and tumor growth of KPAatf and KPAdpgk in Rag2-/- mice after adoptive T cell transfer (ACT) from naïve or tumor-bearing mice on day 4 after tumor injection. Representative data from one of two individual experiments are shown (n = 4 per group per experiment). *p<0.05, ***p<0.001, ****p<0.0001; two-way ANOVA (Tukey) in (B, E), one-way ANOVA (Kruskal–Wallis followed by Dunn’s multiple-comparisons test) in (C, D). Data are shown as mean ± SEM.
-
Figure 2—source data 1
Raw data for Figure 2.
- https://cdn.elifesciences.org/articles/85263/elife-85263-fig2-data1-v3.xlsx
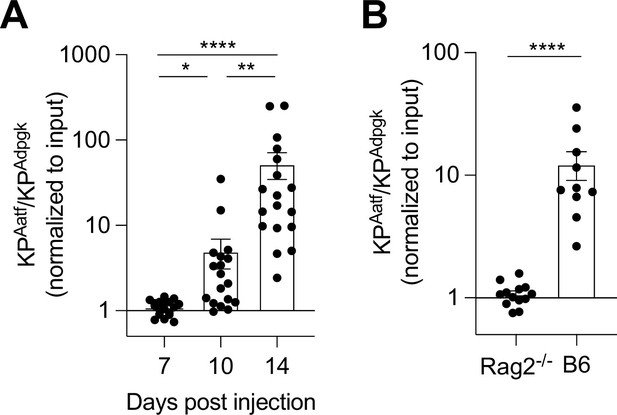
KP-HetHigh tumors are immune edited.
(A, B) 1 × 106 KP-HetHigh cells were implanted in WT or Rag2-/- mice. (A) Tumors from WT mice were collected at days 7, 10, and 14 post tumor inoculation for genomic DNA extraction used for qRT-PCR to determine tumor composition. Pooled data from three independent experiments (pooled n = 18 for each group). (B) Tumors implanted in WT or Rag2-/- mice collected at day 14 or 17 post tumor implantation. Pooled data from three independent experiments (pooled n = 10 for WT, pooled n = 13 for Rag2-/-). *p<0.05, **p<0.01, ****p<0.0001; one-way ANOVA (Kruskal–Wallis followed by Dunn’s multiple comparisons) in (A), Mann–Whitney U in (B).
-
Figure 2—figure supplement 1—source data 1
Raw data for Figure 2—figure supplement 1.
- https://cdn.elifesciences.org/articles/85263/elife-85263-fig2-figsupp1-data1-v3.xlsx
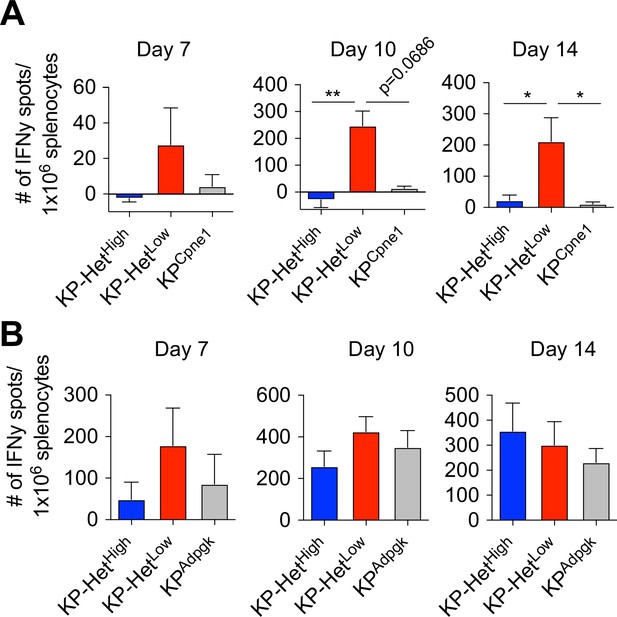
Expansion of neoantigen (NeoAg)-specific T cells directed against weak antigens occurs earlier when they are co-expressed with a stronger antigen.
Splenocytes from tumor-bearing mice expressing Adpgk and/or Cpne1 NeoAgs were used in an IFNγ ELISpot to determine the frequency of NeoAg-specific T cells in the periphery at days 7, 10, and 14 after tumor implantation. Quantification of the (A) Cpne1-specific response and (B) Adpgk-specific response. Pooled data from two independent experiments (n = 3 per group per experiment) in (A, B). *p<0.05, **p<0.01; one-way ANOVA (Kruskal–Wallis followed by Dunn’s multiple-comparisons test) in (A, B). Data are shown as mean ± SEM.
-
Figure 2—figure supplement 2—source data 1
Raw data for Figure 2—figure supplement 2.
- https://cdn.elifesciences.org/articles/85263/elife-85263-fig2-figsupp2-data1-v3.xlsx
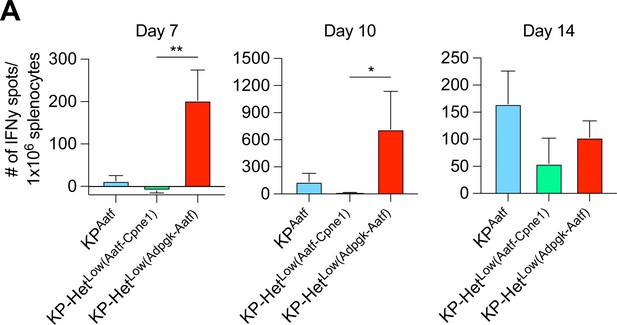
Increased Aatf-specific T cell expansion is not due to increased numbers of antigens expressed in KP-HetLow tumors.
Splenocytes from clonal tumor-bearing mice were used in an IFNγ ELISpot to determine the frequency of neoantigen (NeoAg)-specific T cells in the periphery at days 7, 10, and 14 after tumor implantation. Quantification of the Aatf-specific response. Pooled data from two independent experiments (n = 3 per group per experiment). *p<0.05, **p<0.01; one-way ANOVA (Kruskal–Wallis followed by Dunn’s multiple-comparisons test) in (A). Data are shown as mean ± SEM.
-
Figure 2—figure supplement 3—source data 1
Raw data for Figure 2—figure supplement 3.
- https://cdn.elifesciences.org/articles/85263/elife-85263-fig2-figsupp3-data1-v3.xlsx

Cross-presenting dendritic cells mediate the increased immunogenicity of KP-HetLow tumors.
(A) Tumor growth of KP-HetHigh tumor cells was implanted subcutaneously (s.c.) into Batf3-/-, Rag2-/- and WT mice. Representative data from three independent experiments (n = 5 per group per experiment). (B) Number of cDC1 in KP-HetHigh and KP-HetLow tumors on days 7, 10, and 14 after s.c. implantation. Pooled data from two independent experiments is shown (n = 3 per group per experiment). (C) Proportion of mCherry+ cDC1 in tumor-draining lymph nodes. Pooled data from two independent experiments for days 7 and 10 and three independent experiments for day 14 is shown (n = 3 per group per experiment). (D) Median fluorescence intensity of the mCherry signal of cells from (B). (E) Experimental schematic for (F, G). Tumor cells were irradiated with 40 Gy and 1.5 × 106 total irradiated cells were immediately s.c. injected into mice. A short peptide boost with both peptides and c-di-GMP as adjuvant was given 10 d after and administered s.c. at the base of the day. 21 days after the irradiated cell implantation, spleens were collected for ELISpot. (F) Peripheral Aatf-specific response. Pooled data from three independent experiments are shown (pooled n = 11 or 12 per group). (G) Peripheral Adpgk-specific response. Pooled data from one or three independent experiments are shown (n = 6 for KPAdpgk and pooled n = 12 for remaining groups). *p<0.05, **p<0.01, ****p<0.0001; two-way ANOVA (Tukey) in (A), Mann–Whitney U for each time point between the two tumors was assessed in (B–D), one-way ANOVA (Kruskal–Wallis followed by Dunn’s multiple-comparisons test) in (F, G). Data are shown as mean ± SEM.
-
Figure 3—source data 1
Raw data for Figure 3.
- https://cdn.elifesciences.org/articles/85263/elife-85263-fig3-data1-v3.xlsx
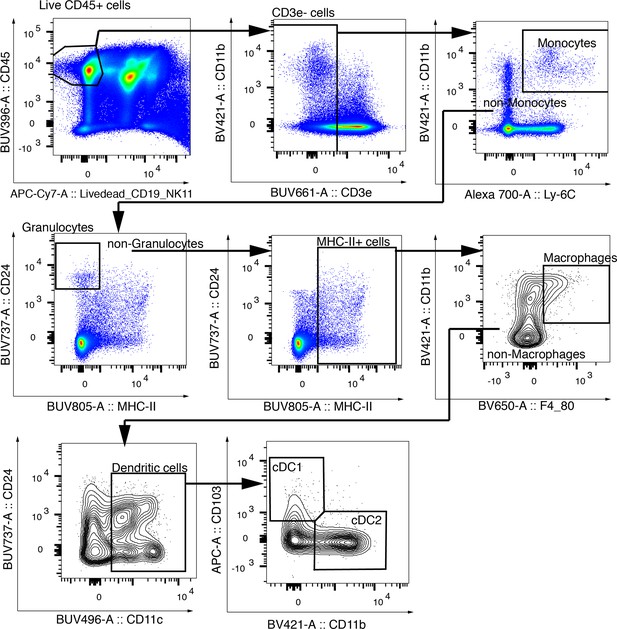
Gating strategy for cDC1.
-
Figure 3—figure supplement 1—source data 1
Raw data for Figure 3—figure supplement 1.
- https://cdn.elifesciences.org/articles/85263/elife-85263-fig3-figsupp1-data1-v3.xlsx
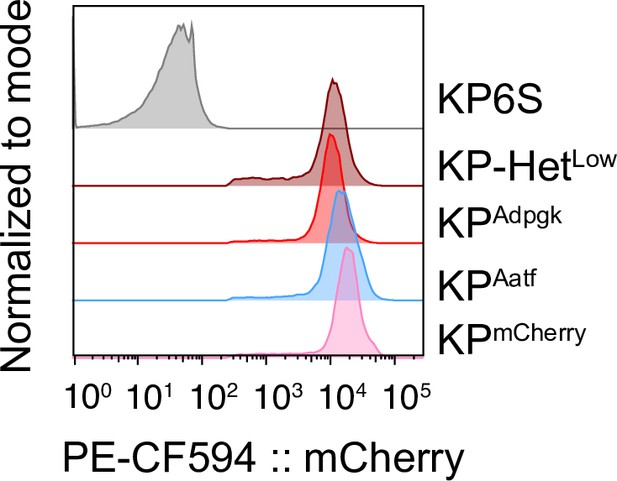
KP cell lines express similar levels of neoantigens (NeoAg).
Representative examples of a flow cytometry analysis of mCherry expression in all cell lines used.
-
Figure 3—figure supplement 2—source data 1
Raw data for Figure 3—figure supplement 2.
- https://cdn.elifesciences.org/articles/85263/elife-85263-fig3-figsupp2-data1-v3.xlsx
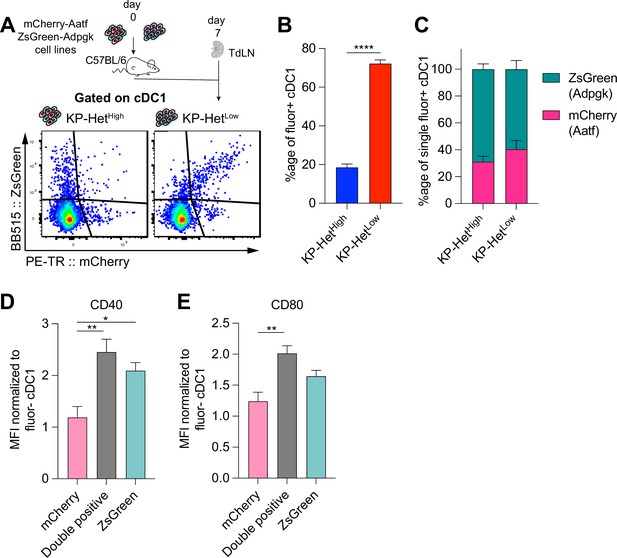
Antigen presentation on dendritic cells in the tumor-draining lymph node mirror antigen expression patterns in the tumor microenvironment (TME).
(A) Experimental schematic for (B, C). KP-HetHigh tumors were composed of KPZsG-Adpgk and KPAatf; KP-HetLow tumors were composed of KP-HetLow(ZsG-Adpgk,Aatf). (B) Quantification of the proportion of cDC1 that are double positive (mCherry+ZsGreen+) in tumors. (C) Proportion of mCherry+ or Zsgreen+ cDC1 in the single positive population. Pooled data from three independent experiments are shown (pooled n = 10 per group) for (B, C). (D) Normalized CD40 median fluorescence intensity for single-positive and double-positive populations. (E) Normalized CD80 median fluorescence intensity for the same sample populations in (D). Pooled data from three independent experiments are shown (pooled n = 13 per group) for (D) and (E). *p<0.05, **p<0.01; one-way ANOVA (Kruskal–Wallis followed by Dunn’s multiple-comparisons test) in (B–E). Data are shown as mean ± SEM.
-
Figure 4—source data 1
Raw data for Figure 4.
- https://cdn.elifesciences.org/articles/85263/elife-85263-fig4-data1-v3.xlsx
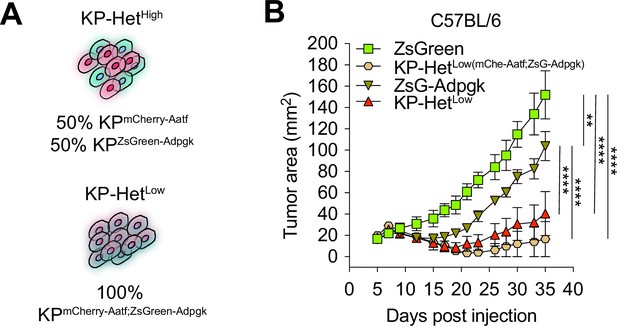
Clonal expression of Adpgk and Aatf neoantigens (NeoAgs) results in increased immunogenicity regardless of linked or separate expression of NeoAgs in the same cell.
(A) Schematic of cells generated used in (B). (B) Tumor growth in WT mice. n = 3 per group. **p<0.01, ****p<0.0001; two-way ANOVA (Tukey) in (A). Data are shown as mean ± SEM.
-
Figure 4—figure supplement 1—source data 1
Raw data for Figure 4—figure supplement 1.
- https://cdn.elifesciences.org/articles/85263/elife-85263-fig4-figsupp1-data1-v3.xlsx
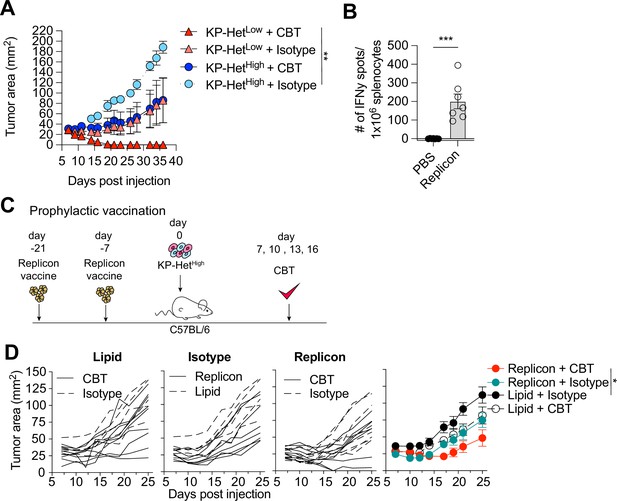
Prophylactic mRNA replicon vaccination increases response to checkpoint blockade therapy in KP-HetHigh tumors.
(A) Tumor growth of KP-HetHigh and KP-HetLow WT mice treated with checkpoint blockade immunotherapy (CBT) or control. 100 µg of each antibody (CBT or isotype control) was administered intraperitoneally (i.p.) on days 7, 10, 13, and 16 after implantation. Representative data from one of two individual experiments are shown (n = 3 per group per experiment). (B) IFNγ ELISpot using splenocytes from mice vaccinated with replicons expressing Aatf. Pooled data from two independent experiments (n = 3 or 4 per group per experiment). (C) Experimental schematic for prophylactic vaccination in (C). Three weeks before tumor-challenge mice are initially vaccinated, replicons are administered intramuscularly (i.m.). Animals are boosted 1 wk before challenge. CBT is administered (i.p.) on days 7, 10, 13, and 16 following subcutaneous (s.c.) implantation of KP-HetHigh. (D) KP-HetHigh outgrowth in WT mice. Individual traces for mice dosed with a lipid-only control and treated with CBT or an isotype antibody control, treated only with isotype antibody control and dosed with the replicon or lipid only control, and vaccinated with the replicon and treated with CBT or an isotype control (left to right). Far-right plot is the averaged results. Representative data from three independent experiments (n = 5–10 per group per experiment). *p<0.05, **p<0.01, ***p<0.001; two-way ANOVA (Tukey) in (A) and (D), Mann–Whitney U in (B). Data are shown as mean ± SEM.
-
Figure 5—source data 1
Raw data for Figure 5.
- https://cdn.elifesciences.org/articles/85263/elife-85263-fig5-data1-v3.xlsx
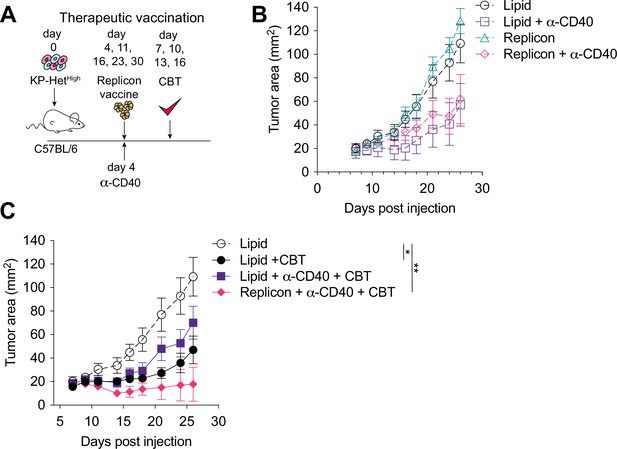
Therapeutic mRNA replicon vaccination synergizes with checkpoint blockade immunotherapy (CBT) and CD40 agonism in KP-HetHigh tumors.
(A) Experimental schematic for therapeutic vaccination in (B, C). Animals were implanted with 1 × 106 KP-HetHigh tumor cells implanted subcutaneously (s.c.). Tumor-bearing mice were vaccinated with replicon vaccine (i.m.) on day 4 post tumor inoculation and continually vaccinated every following week. Anti-CD40 antibody (100 µg) was given with the first vaccination dose only. Dual CBT was administered intraperitoneally (i.p.) on days 7, 10, 13, and 16 post tumor inoculation. (B) Tumor growth of KP-HetHigh treated with replicons or lipid only with or without anti-CD40 antibody. (C) Tumor growth of KP-HetHigh treated with replicons or lipid only with or without anti-CD40 and CBT. n = 5 per group. The dotted line is the same cohort observed in (B) of mice administered with only lipid and isotype control antibodies. *p<0.05, **p<0.01; two-way ANOVA (Tukey) in (B, C). Data are shown as mean ± SEM.
-
Figure 6—source data 1
Raw data for Figure 6.
- https://cdn.elifesciences.org/articles/85263/elife-85263-fig6-data1-v3.xlsx
Tables
Amino acid sequences of wildtype and NeoAg.
| Name | Wildtype | Mutant | |||||
|---|---|---|---|---|---|---|---|
| Peptide sequence | Sequence position | Binding prediction | Predicted affinity (IC50) | Peptide sequence | Binding prediction | Predicted affinity (IC50) | |
| Adpgk | ASMTNRELM | 298–307 | Db | 6.21 | ASMTNMELM | Db | 4.29 |
| Aatf | MAPIDHTAM | 493–501 | Db | 297.14 | MAPIDHTTM | Db | 90.16 |
| Cpne1 | SSPDSLHYL | 298–307 | Db | 764.37 | SSPYSLHYL | Db | 182.34 |
Additional files
-
Supplementary file 1
List of antibodies used in the study.
- https://cdn.elifesciences.org/articles/85263/elife-85263-supp1-v3.xlsx
-
MDAR checklist
- https://cdn.elifesciences.org/articles/85263/elife-85263-mdarchecklist1-v3.pdf





