Multiple NTS neuron populations cumulatively suppress food intake
Figures
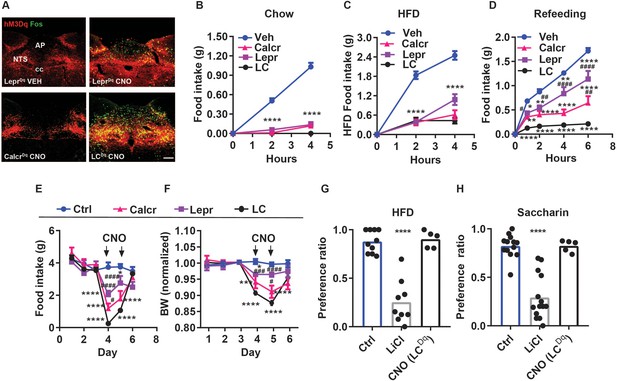
Additive suppression of food intake with combined activation of NTSCalcr and NTSLepr neurons.
(A) Representative images showing mCherry-IR (hM3Dq, red) and FOS-IR (green) in the NTS (approximately Bregma –7.56 mm) of LeprDq, CalcrDq and LCDq mice following treatment with saline (LeprDq VEH) or CNO (LeprDq CNO, CalcrDq CNO and LCDq CNO IP, 1 mg/kg) for 2 hr before perfusion. NTS: nucleus of the solitary tract, AP: area postrema, cc: central canal. All images were taken at the same magnification; scale bar equals 150 µm. (B–D) Food intake in chow-fed LeprDq (Lepr), CalcrDq (Calcr) and LCDq (LC) mice over the first 4 hr of the dark phase following vehicle (Veh) or CNO injection (IP, 1 mg/kg) when provided with chow (B, n=25 for Veh group; n=20 for both Lepr and Calcr groups; n=8 for LC group) or HFD (C, n=21 for Veh group; n=6 for Lepr group; n=7 for Calcr group; n=8 for LC group). (D) Food intake for the same groups of mice over the first 6 hours of refeeding during the light cycle following an overnight fast (D, n=23 for Veh group; n=8 for Lepr group; n=7 for Calcr group; n=7 for LC group) following with CNO (IP, 1 mg/kg) or vehicle (Veh). (E–F) Control (Ctrl; AAVGFP or AAVcre-GFP-injected, n=5) or LeprDq (Lepr, n=5), CalcrDq (Calcr, n=5) and LCDq (LC, n=8) mice were treated with vehicle for three baseline days, followed by 2 days of twice daily treatment with CNO (IP 1 mg/kg), followed by two additional days of Veh treatment. Daily food intake (E) and body weight (shown as change from baseline) (F). Vehicle and CNO treatment are denoted on the graphs. (G, H) CTA assays: Mice were treated with vehicle (Veh, n=10), LiCl (IP, 126 mg/kg, n=9), or CNO (IP, 1 mg/kg, n=5) during exposure to a novel tastant (HFD) (G) or saccharin (H) paired with vehicle (Veh, n=13), LiCl (IP, 126 mg/kg, n=14), or CNO (IP, 1 mg/kg, n=5). Shown is mean +/-SEM; Two-way ANOVA, sidak’s multiple comparisons test was used; *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001 vs vehicle or Ctrl; #p<0.05, ##p<0.01, ###p<0.001, ####p<0.0001 vs LC.
-
Figure 1—source data 1
Data for Figure 1 panels B-H.
- https://cdn.elifesciences.org/articles/85640/elife-85640-fig1-data1-v3.xlsx
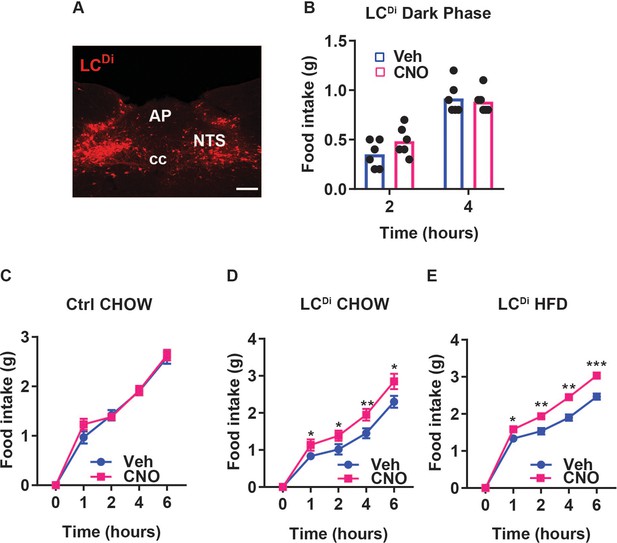
Inhibiting NTSLC neurons increases food intake.
(A) Representative NTS image showing dsRed-IR (red, mCherry) in the NTS of LeprCre;CalcrCre mice injected with mCherry-expressing AAVFlex-hM4Di (LCDi mice) (approximately Bregma –7.56 mm). NTS: the nucleus of the solitary tract, AP: area postrema, cc: central canal; scale bar equals 150 µm. (B) Food intake in LCDi mice during treatment with vehicle (Veh) or CNO injection (IP, 1 mg/kg) at the onset of dark cycle, n=6 in each group. (C) Food intake during the first 6 hours of refeeding in the light cycle following an overnight fast for control (C, n=6 per condition) or LCDi (D, n=6 per condition) animals fed with chow and for LCDi animals fed with HFD (E, n=6 per group) during treatment with CNO (IP, 1 mg/kg) or Veh. Shown is mean +/-SEM. Two-way ANOVA, sidak’s multiple comparisons test was used, *p<0.05, **p<0.01, ***p<0.001 vs Veh.
-
Figure 1—figure supplement 1—source data 1
Source data for Figure 1—figure supplement 1 panels B-E.
- https://cdn.elifesciences.org/articles/85640/elife-85640-fig1-figsupp1-data1-v3.xlsx
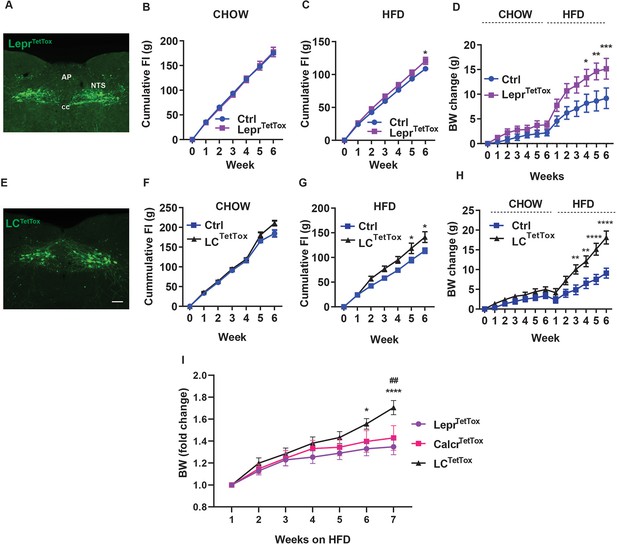
Silencing NTSLepr neurons or NTSLCS neurons exacerbates diet-induced obesity (DIO).
Representative images showing GFP-IR (green) from in LeprTetTox (A) and LCTetTox (E) mice (approximately Bregma –7.56 mm). All images were taken at the same magnification; scale bar equals 150 µm. NTS: nucleus of the solitary tract, AP: area postrema, cc: central canal. B-D, F-H show cumulative food intake during chow (B, F) and HFD feeding (C, G) and body weight (change from baseline) (D, H) for control (Ctrl; AAVFLEX-hM3Dq-injected), LeprTetTox (B–D) and LCTetTox (F–H) mice following VSG, during which time they were fed with chow for 5–6 weeks and HFD for an additional 6 weeks. n=7 each group for B and C, n=8 each group for D for Ctrl and LeprTetTox group; n=7 for Ctrl and n=8 for LCTetTox group in F and G, n=11 for Ctrl and n=12 for LCTetTox group in H. (I) Shows weight gain from the onset of HFD feeding for LeprTetTox (from D) and LCTetTox mice (from H) in comparison to previously-published CalcrTetTox mice (Cheng et al., 2020a). Shown are mean +/-SEM. Two-way ANOVA, sidak’s multiple comparisons test was used; *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001 vs Ctrl, except in I, where these refer to the comparison between LeprTetTox and LCTetTox; ##p<0.01 between CalcrTetTox and LCTetTox.
-
Figure 2—source data 1
Source data for Figure 2 Panels B-D, F-I.
- https://cdn.elifesciences.org/articles/85640/elife-85640-fig2-data1-v3.xlsx
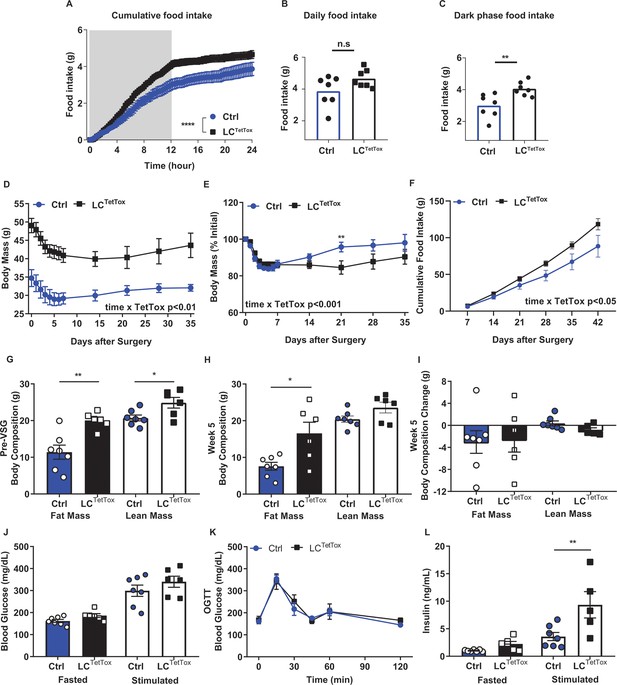
Silencing NTSLC neurons increases food intake and body weight but fails to abrogate the weight loss effects of VSG.
(A–C) Cumulative food intake (A), daily food intake (B) and dark phase food intake (C) were continuously monitored over 24 hours in a TSE system during the 7th week after surgery with control (Ctrl; AAVFLEX-Dq-injected, n=7) and LCTetTox (n=7) animals. (D–L) Response to VSG for Ctrl and LCTetTox mice. Body mass (absolute (D), and %initial (E)), cumulative food intake (F), body composition before VSG (G), 5 weeks after VSG (H) and change in body composition (I) are shown for Ctrl and LCTetTox mice following VSG. (J–L) Response to an oral glucose load (J, 2 g/kg, gavage), along with fasting and stimulated (10 min after gavage) glucose (K) and insulin (L) concentrations are shown for Ctrl and LCTetTox mice following VSG. Shown is mean +/-SEM. For A-C, two-way ANOVA, sidak’s multiple comparisons test was used. For D–L, data was analyzed using RM-ANOVA or Student’s T-test; **p<0.01, ****p<0.0001 vs Ctrl. Mice were a mixture of males and females; Ctrl, n=7; LCTetTox, n=6.
-
Figure 2—figure supplement 1—source data 1
Source data for Figure 2—figure supplement 1, Panels A-L.
- https://cdn.elifesciences.org/articles/85640/elife-85640-fig2-figsupp1-data1-v3.xlsx
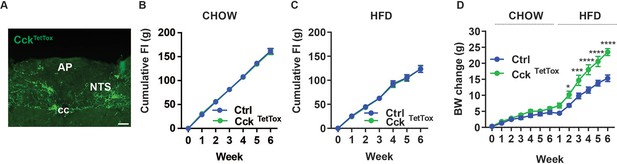
Silencing NTSCck neurons exacerbates DIO.
(A) Representative images showing GFP-IR (green) in the NTS of CckTetTox mice (approximately Bregma –7.56 mm). Images were taken at the same magnification; scale bar equals 150 µm. NTS: nucleus of the solitary tract, AP: area postrema, cc: central canal. (B–D) Cumulative food intake during chow (B) and HFD (C) feeding and body weight (D, change from baseline) is shown for control (Ctrl; AAVFLEX-hM3Dq-injected) and CckTetTox mice following surgery, during which time they were fed with chow for 6 weeks and the HFD for an additional 6 weeks. n=5 each group for B, n=6 for Ctrl and n=7 for CckTettox group in C, n=11 for Ctrl group and n=10 for CckTetTox group in D. Shown is mean +/-SEM. Two-way ANOVA, sidak’s multiple comparisons test was used, *p<0.05, ****p<0.0001 vs Ctrl.
-
Figure 3—source data 1
Source Data for Figure 3, panels B-D.
- https://cdn.elifesciences.org/articles/85640/elife-85640-fig3-data1-v3.xlsx
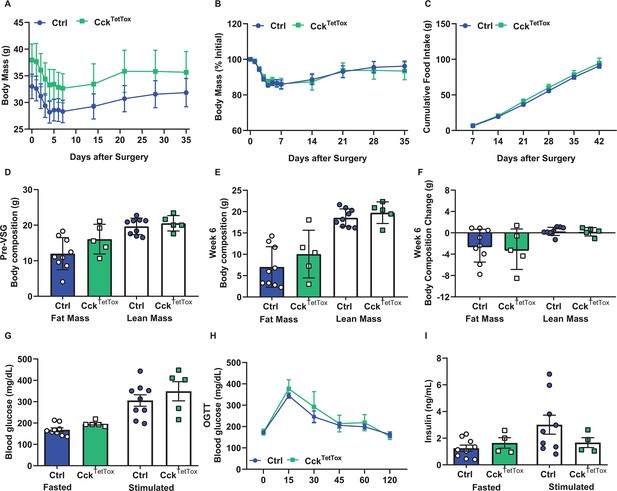
Response of CckTetTox mice to VSG.
Body mass (absolute (A), and %initial (B)), cumulative food intake (C), body composition before VSG (D), 5 weeks after VSG (E) and change in body composition (F) are shown for control (Ctrl; AAVFLEX-Dq-injected) and CckTetTox mice following VSG. (G–I) Response to an oral glucose load (G, 2 g/kg, gavage), along with fasting and stimulated (time) glucose (H) and insulin (I) concentrations are shown for Ctrl and CckTetTox mice following VSG. Shown is mean +/-SEM. Data was analyzed using RM-ANOVA or Student’s T-test. Mice were a mixture of males and females (Ctrl, n=9; CckTetTox, n=4–5).
-
Figure 3—figure supplement 1—source data 1
Source data for Figure 3—figure supplement 1, panels A-I.
- https://cdn.elifesciences.org/articles/85640/elife-85640-fig3-figsupp1-data1-v3.xlsx

NTSLCK neuron activation decreases food intake and promotes a CTA.
(A) Representative images showing mCherry-IR (hM3Dq, red) and FOS-IR (green) in the NTS (approximately Bregma –7.56 mm) of LCKDq mice following treatment with CNO (IP, 1 mg/kg) 2 hr before perfusion. Scale bar equals 150 µm. NTS: nucleus of the solitary tract, AP: area postrema, cc: central canal. (B–D) Food intake in LCKDq mice over the first 4 hr of the dark phase during expose to chow (B) or HFD (C) (n=9 per group), and during the first 6 hr of refeeding in the light cycle following an overnight fast (D, n=7 per group) during treatment with CNO (IP, 1 mg/kg) or vehicle. (E–F) Control (Ctrl; AAVGFP-injected, n=7) or LCKDq mice (n=7) mice were treated vehicle (Veh) for 1 days, followed by 2 days with CNO (1 mg/kg, IP, twice per day) and daily food intake (E) and body weight (change from baseline) (F) were determined. Veh and CNO treatment are denoted on the graphs. (G, H) Ctrl (uninjected littermates) or LCKDq mice were treated with Veh, LiCl, or CNO (IP, 1 mg/kg) during exposure to a novel tastant (HFD (G) or saccharin (H)); n=6 per group. Shown is mean +/-SEM; Two-way ANOVA, sidak’s multiple comparisons test was used in all figures, except panels G and H where unpaired T test was used; *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001 vs Veh or Ctrl.
-
Figure 4—source data 1
Source data for Figure 4, Panels B-H.
- https://cdn.elifesciences.org/articles/85640/elife-85640-fig4-data1-v3.xlsx
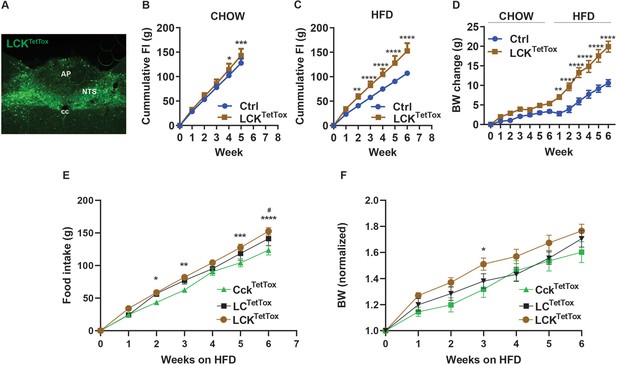
Silencing of NTSLCK neurons increases food intake and body weight.
(A) Representative image showing GFP-IR (green) in the NTS of LCKTetTox mice (approximately Bregma –7.56 mm). Scale bar equals 150 µm. NTS: the nucleus of the solitary tract, AP: area postrema, cc: central canal. (B–D) Cumulative food intake in response to chow (B, n=8 per group) and HFD (C, n=5 for control (Ctrl; AAVFLEX-hM3Dq-injected) group and n=8 for LCKTetTox group) and body weight (D, change from baseline; n=6 for Ctrl group and n=8 for LCKTetTox group) is shown for Ctrl and LCKTetTox mice following surgery, during which time they were fed chow for 6 weeks and HFD for an additional 6 weeks. (E–F) Comparisons of cumulative HFD food intake (E) and body weight (normalized to baseline) for CckTetTox (from Figure 3C), LCTetTox (from Figure 2G), and LCKTetTox (from panel C) mice. Shown is mean +/-SEM; Two-way ANOVA, sidak’s multiple comparisons test was used; *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001 vs Ctrl except E and F, for which these indicate differences between CckTetTox and LCKTetTox; #p<0.05 for CckTetTox vs LCTetTox.
-
Figure 5—source data 1
Source data for Figure 5, panels B-F.
- https://cdn.elifesciences.org/articles/85640/elife-85640-fig5-data1-v3.xlsx
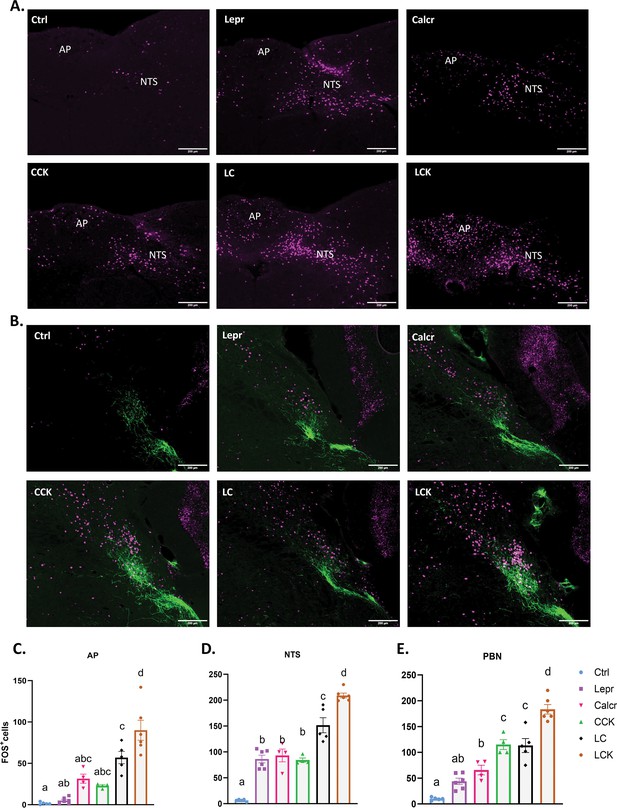
FOS-IR in the AP, NTS, and PBN following activation of single or multiple populations of NTS neurons.
Control (Ctrl; AAVGFP-injected), LeprDq, CalcrDq, CCKDq, LCDq, and LCKDq animals were fasted overnight, injected with CNO (1 mg/kg) and perfused 2 hr later. Brains were processed and stained for FOS (or FOS and CGRP)-IR. (A) Representative images of the AP and NTS from the indicated animal type showing FOS-IR (magenta) (approximately Bregma –7.56 mm). (B) Representative images of the PBN (approximately Bregma –5.20 mm) from the indicated animal type showing FOS-IR (magenta) and CGRP-IR (green). For A-B scale bars represent 200 μm. (C-E) Quantification of FOS-IR in the indicated regions for CNO-treated animals of each type. All bars, mean +/-SEM is shown, along with the distribution of values for each bar. CalcrDq, CckDq, n=4 each; Ctrl, LCDq, n=5 each; LeprDq, LCKDq, n=6 each. Bars with the same letter are not different by ANOVA (i.e. bars without overlapping letters are different by ANOVA).
-
Figure 6—source data 1
Source data for Figure 6, panels C-E.
- https://cdn.elifesciences.org/articles/85640/elife-85640-fig6-data1-v3.xlsx
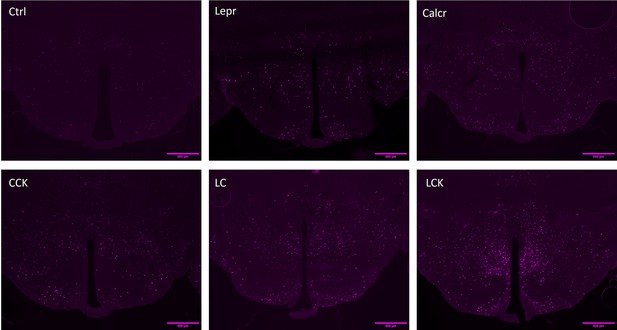
FOS-IR in the DMH following activation of single or multiple populations of NTS neurons.
Control (Ctrl; AAVGFP-injected), LeprDq, CalcrDq, CCKDq, LCDq, and LCKDq animals were fasted overnight, injected with CNO (1 mg/kg) and perfused 2 hr later. Brains were processed and stained for FOS-IR. Shown are representative images of FOS-IR (magenta) in the DMH (approximately Bregma –1.94 mm) for the indicated animal type. Scale bars represent 500 μm.
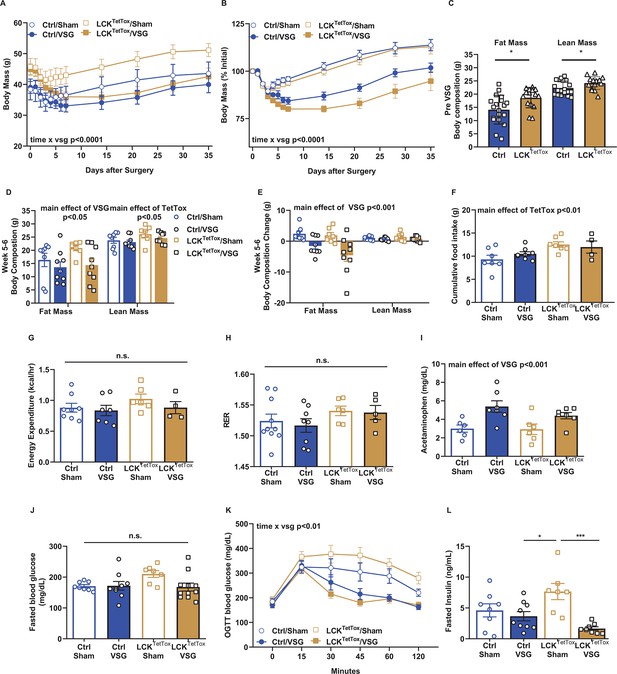
Appropriate response to VSG for LCKTetTox mice.
(A–E) Shown is body weight (absolute (A) and %initial (B)) and body composition before (C) and 6 weeks after (D) VSG, along with change in body composition from baseline (E) for body mass in control (Ctrl; AAVFLEX-hM3Dq-injected or uninjected wild-type) and LCKTetTox groups after VSG or sham surgery. (F–H) A subset of mice was placed in metabolic chambers for 7 days (3 days acclimation, 4 days data analysis) for the determination of food intake (F, cumulative over the 4-day data collection period) energy expenditure (G), and RER (H). (I) Gastric emptying rate assessed by acetaminophen appearance in the plasma following gavage. (J–L) Fasting blood glucose (J), oral glucose tolerance (2 g/kg) (K), and fasting insulin are shown for all groups. Data were analyzed using RM-ANOVA or Student’s T-test and are reported as mean +/-SEM. Males mice ctrl/sham (n=6–8), ctrl/VSG (n=7–9), LCKTetTox/sham (n=6–7), LCKTetTox/VSG (n=4–9). *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001 for the indicated comparisons.
-
Figure 6—figure supplement 2—source data 1
Source data for Figure 6—figure supplement 2, panels A-L.
- https://cdn.elifesciences.org/articles/85640/elife-85640-fig6-figsupp2-data1-v3.xlsx
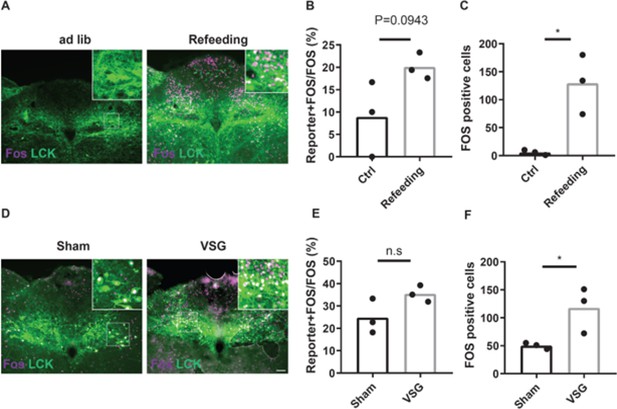
Activation of NTSLCK and other NTS neurons in response to refeeding and VSG.
LeprCre;CalcrCre;CckCre mice were injected with cre-dependent AAV reporters (LCKReporter mice). LCKReporter mice were treated as described below and stained for the appropriate reporter (LCK, green) and FOS (magenta). (A–C): LCKReporter mice were fed ab libitum (Ctrl) or overnight fasted (16 hr) and refed (Refeeding) prior to perfusion. (A) Representative images of the NTS (approximately Bregma –7.56 mm). (B–C) Quantification of Reporter +FOS colocalized neurons as a percentage of total FOS neurons (B) and total FOS neurons (C) per section. (D–F) LCKReporter mice were subjected to sham surgery (Sham) or VSG. After recovery, they were fasted for 4 hr during the light cycle and gavaged with a glucose load prior to perfusion. (D) Shows representative images of the NTS (approximately Bregma –7.56 mm). (E–F) Quantification of Reporter +FOS colocalized neurons as a percentage of total FOS neurons (E) and total FOS neurons (F) per section. Shown is mean +/-SEM; n=3 mice per group. Student’s unpaired t-test was performed. n.s=not significantly different; * p<0.05 vs Ctrl or Sham. Scale bar equals 150 µm.
-
Figure 6—figure supplement 3—source data 1
Source Data for Figure 6—figure supplement 3, panels B-C, E-F.
- https://cdn.elifesciences.org/articles/85640/elife-85640-fig6-figsupp3-data1-v3.xlsx
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Strain, strain background (mus musculus) | CalcrCre; C57BL6/J | Jackson Lab Strain | #037028 | |
| Strain, strain background (mus musculus) | LeprCre: C57Bl6/J | Jackson Lab Strain | #17527 | |
| Strain, strain background (mus musculus) | CckCre; C57Bl6/J | Jackson Lab Strain | #012706 | |
| Antibody | anti-FOS (rabbit monoclonal) | Cell Signaling Technology | #2250 | IF: 1:1000 |
| Antibody | anti-GFP (chicken polyclonal) | Aves Laboratories | GFP1020 | IF: 1:1000 |
| Antibody | anti-dsRed (rabbit polyclonal) | Takara | 632496 | IF: 1:1000 |
| Recombinant DNA reagent | AAVFLEX-hM3Dq | DOI: 10.1172/JCI46229 | ||
| Recombinant DNA reagent | AAVFLEX-TetTox-GFP | DOI: 10.1038/nn.4574 | ||
| Recombinant DNA reagent | AAVFLEX-hM4Di | DOI: 10.1172/JCI46229 | ||
| Recombinant DNA reagent | AAVCre-GFP | DOI: 10.1073/pnas.042678699 | ||
| Recombinant DNA reagent | AAVGFP | DOI: 10.1073/pnas.042678699 | ||
| Chemical compound, drug | CNO | Tocris Bioscience | #4936 |





