Systemic pharmacological suppression of neural activity reverses learning impairment in a mouse model of Fragile X syndrome
Figures
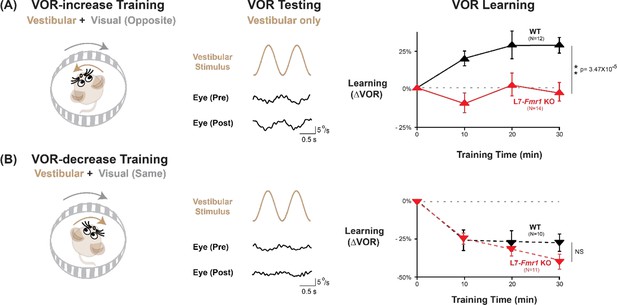
VOR-increase learning is impaired in L7-Fmr1 KO mice with enhanced cerebellar LTD.
(A) Training to increase the VOR. Left, VOR-increase training paired a vestibular stimulus (1 Hz sinusoidal rotation about an earth-vertical axis, brown) with oppositely directed visual stimulus motion (grey). Middle, Example raw eye velocity responses (black) to the vestibular stimulus alone in the dark, that is, the VOR, measured Pre and Post VOR-increase training. Right, Average learned change in the amplitude of the VOR relative to pre-training (upward triangles), measured in the dark after each 10 min VOR-increase training block in the L7-Fmr1 KO (red) and WT mice (black). (B) Training to decrease the VOR. Left, VOR-decrease training paired a vestibular stimulus (1 Hz sinusoidal rotation) with visual stimulus motion in the same direction. Middle, Example VOR responses in the dark, measured Pre and Post VOR-decrease training. Right, VOR-decrease learning (downward triangles). NS = not significant. In this and all figures, values plotted are mean ± SEM.
-
Figure 1—source data 1
VOR-increase learning is impaired in L7-Fmr1 KO mice with enhanced cerebellar LTD.
- https://cdn.elifesciences.org/articles/92543/elife-92543-fig1-data1-v1.xlsx
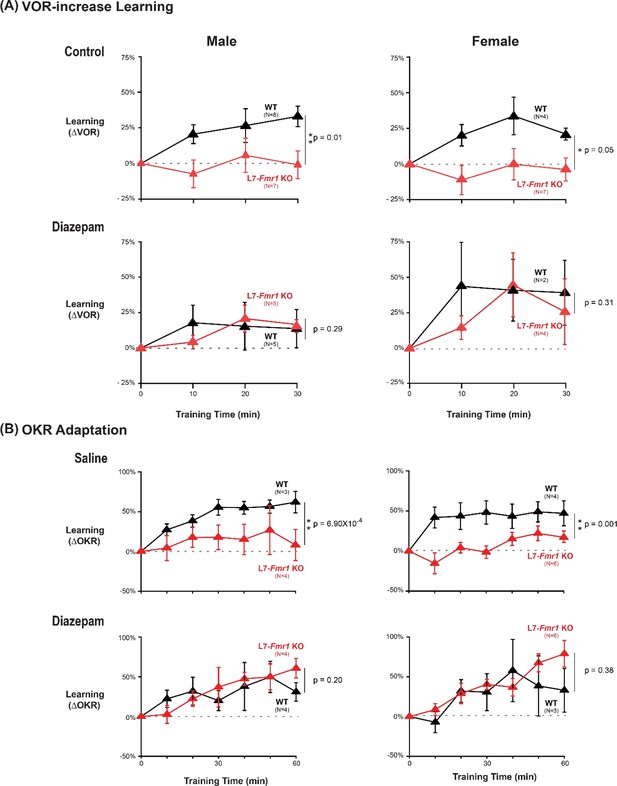
Similar oculomotor learning impairments and efficacy of diazepam pretreatment in male and female L7-Fmr1 KO mice.
(A) VOR-increase training. Top Left, In male L7-Fmr1 KO mice (red), VOR-increase learning was impaired relative to male WT (black) (p=0.01, 30 min, Tukey). Top Right, In female L7-Fmr1 KO mice (red), VOR-increase learning was impaired relative to female WT (black) (p=0.05, 30 min, Tukey). Bottom Left, 18–24 hours after diazepam administration, male L7-Fmr1 KO mice exhibited VOR-increase learning indistinguishable from that of male WT (p=0.290, 30 min, Tukey). Bottom Right, 18–24 hr after diazepam administration, female L7-Fmr1 KO mice exhibited VOR-increase learning indistinguishable from that of female WT (p=0.31, 30 min, Tukey). (B) OKR adaptation training. Top Left, In male L7-Fmr1 KO mice, OKR adaptation was impaired relative to male WT (p=6.90 × 10–4, 30 min, Tukey). Top Right, In female L7-Fmr1 KO mice, OKR adaptation was impaired relative to female WT (p=0.001, 30 min, Tukey). Bottom Left, 18–24 hr after diazepam administration, male L7-Fmr1 KO mice exhibited OKR adaptation indistinguishable from that of male WT (p=0.20, 30 min, Tukey). Bottom Right, 18–24 hr after diazepam administration, female L7-Fmr1 KO mice exhibited OKR adaptation indistinguishable from that of female WT (p=0.38, 30 min, Tukey).
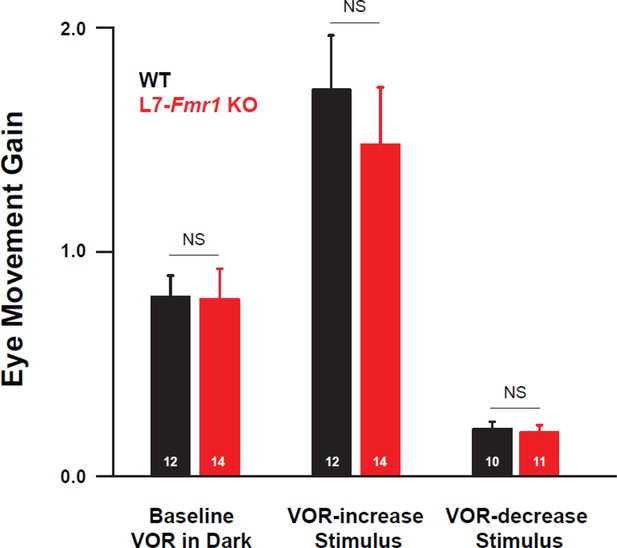
Baseline oculomotor performance of L7-Fmr1 KO mice was indistinguishable from WT.
The gain of the eye movement responses (ratio of eye movement amplitude to vestibular stimulus amplitude; see Methods) of L7-Fmr1 KO mice (red) was not significantly different from that of WT mice (black) during baseline tests of the VOR in the dark before training (left; p=0.95, two sample t-test) or during the first 45 s of the paired presentation of visual and vestibular stimuli used for VOR-increase training (middle; p=0.50, two sample t-test) or for VOR-decrease training (right; p=0.76, two sample t-test). Number of mice tested is indicated in each bar.
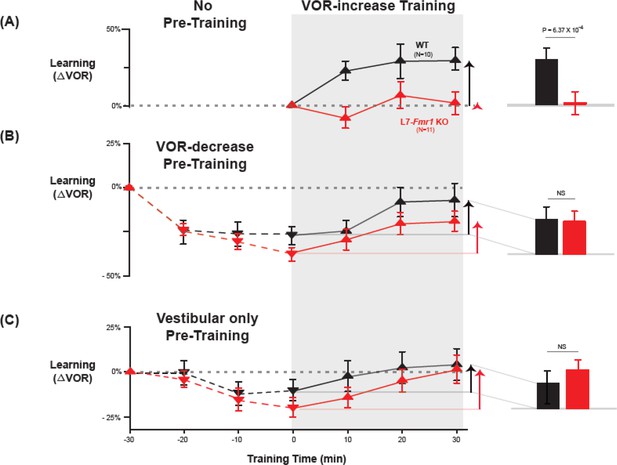
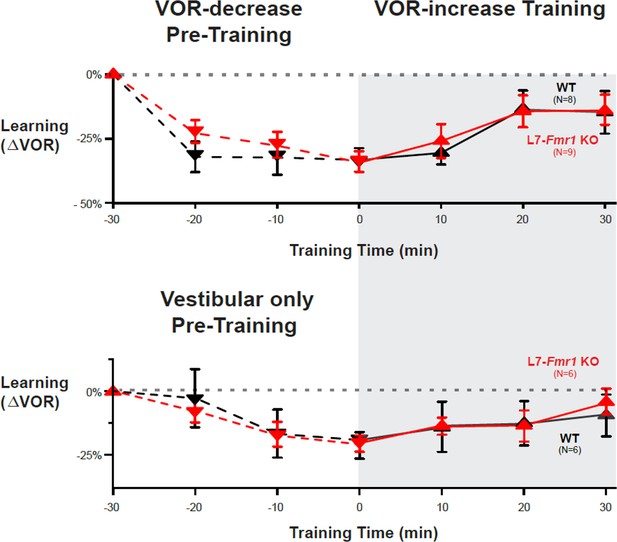
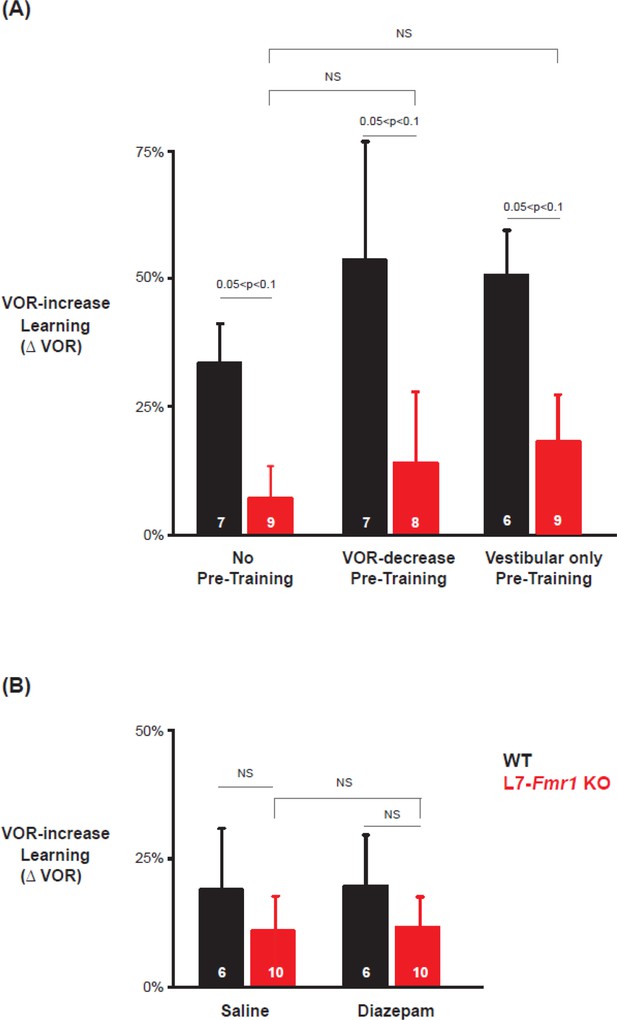
Behavioral pre-training rescued learning impairment of L7-Fmr1 KO mice with enhanced associative LTD.
Associative VOR-increase learning (shaded area and bar graphs), without pre-training (A), after VOR-decrease pre-training (B), and after Vestibular only pre-training (C). (A) Learned change in the VOR response measured in the dark after each 10 min block of VOR-increase training in the subset of L7-Fmr1 KO (red) and WT (black) mice from Figure 1A that were also tested after pre-training. (B) Changes in the VOR measured in the dark after each block of VOR-decrease pre-training (downward triangles, dashed lines) and then subsequent VOR-increase training (upward triangles, solid lines). (C) Changes in the VOR measured in the dark after each block of Vestibular only pre-training (downward triangles, dashed lines) and then VOR-increase training (upward triangles, solid lines). Right, Arrows and bars graphs show the total change in the VOR induced by 30 min of VOR-increase training (training time = 30) compared with just before VOR-increase training (training time = 0).
-
Figure 2—source data 1
Behavioral pre-training rescued learning impairment of L7-Fmr1 KO mice with enhanced associative LTD.
- https://cdn.elifesciences.org/articles/92543/elife-92543-fig2-data1-v1.xlsx
Data from Figure 2 were subsampled to compare VOR-increase learning in subpopulations of mice matched for the mean learned decrease in the VOR during pre-training.
Subsampling was done by eliminating the WT mice (black) with the smallest decrease and L7-Fmr1 KO mice (red) with the largest decrease in the VOR measured after 30 min of pre-training (just before the start of VOR-increase training), until the mean values in the two populations were within 2%. In these sub-sampled populations, the amount of VOR-increase learning was not significantly different between the L7-Fmr1 KO and WT mice after VOR-decrease pre-training (top; p=0.74, L7-Fmr1 KO mice vs. WT, 30 min, Tukey) or after Vestibular only pre-training (bottom; p=0.40, L7-Fmr1 KO mice vs. WT, 30 min, Tukey), as also observed in the full samples.
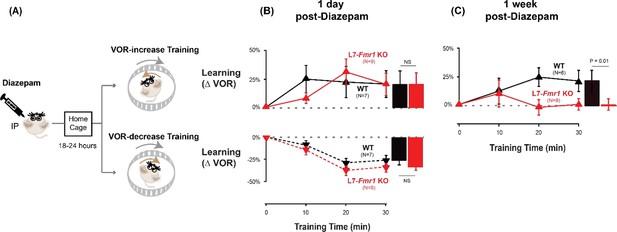
Diazepam pre-treatment rescued learning impairment of L7-Fmr1 KO mice with enhanced associative LTD.
(A) Mice were given an intraperitoneal (IP) injection of diazepam (0.5 mg/kg) and then returned to the home cage for 18–24 hr, followed by VOR-increase (top) or VOR-decrease (bottom) training. (B) Top, VOR-increase learning 1 day (18–24 hr) after diazepam administration in L7-Fmr1 KO (red upward triangles) and WT mice (black upward triangles).Bottom, VOR-decrease learning (downward triangles) 1 day after diazepam. (C) VOR-increase learning in the same mice as in (B), 1 week after diazepam treatment, and 18–24 hr after IP saline injection.
-
Figure 3—source data 1
Diazepam pre-treatment rescued learning impairment of L7-Fmr1 KO mice with enhanced associative LTD.
- https://cdn.elifesciences.org/articles/92543/elife-92543-fig3-data1-v1.xlsx
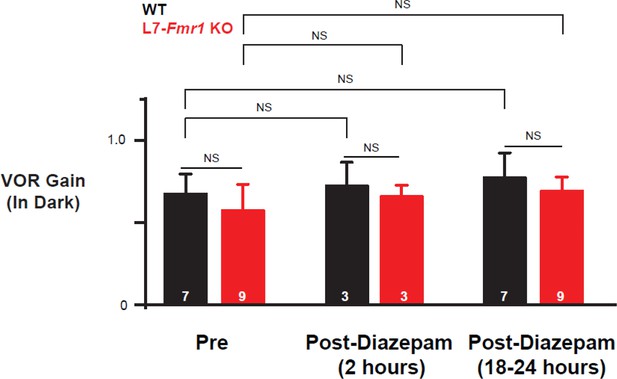
Diazepam did not affect baseline VOR performance.
The gain of the VOR (ratio of eye velocity to vestibular stimulus velocity) was measured in the dark in L7-Fmr1 KO (red) and WT (black) mice before (Pre), 2 hr after (Post-diazepam (2 hr)) and 18–24 hr after (Post-diazepam (18–24 hr)) an IP injection of diazepam (0.5 mg/kg). There was no effect of diazepam on the gain of the VOR in L7-Fmr1 KO mice (red; p=0.72, Pre vs. 2 hr Post Diazepam; p=0.77, Pre vs. 18–24 hr Post-diazepam; Tukey) or WT mice (black; p=0.99, Pre vs. 2 hr Post diazepam; p=0.36, Pre vs. 18–24 hr Post-diazepam; Tukey). Moreover, the gain of the VOR of L7-Fmr1 KO mice was not significantly different from that of WT mice during baseline tests of the VOR in the dark before diazepam administration Pre (left; p=0.79, Tukey), Post-diazepam (2 hr) (middle; p=0.77, Tukey) and Post-diazepam (18–24 hr) (right; p=0.97, Tukey). The 2 hr and 18–24 hr VOR performance measurements were made just before the VOR-increase training sessions (training time = 0) shown in Figure 3—figure supplement 2B, and Figure 3B top, respectively. The Pre VOR-performance measurements were made just before the VOR-increase training sessions shown in Figure 1A, right for the subset of mice that were also tested 1 day after diazepam administration.
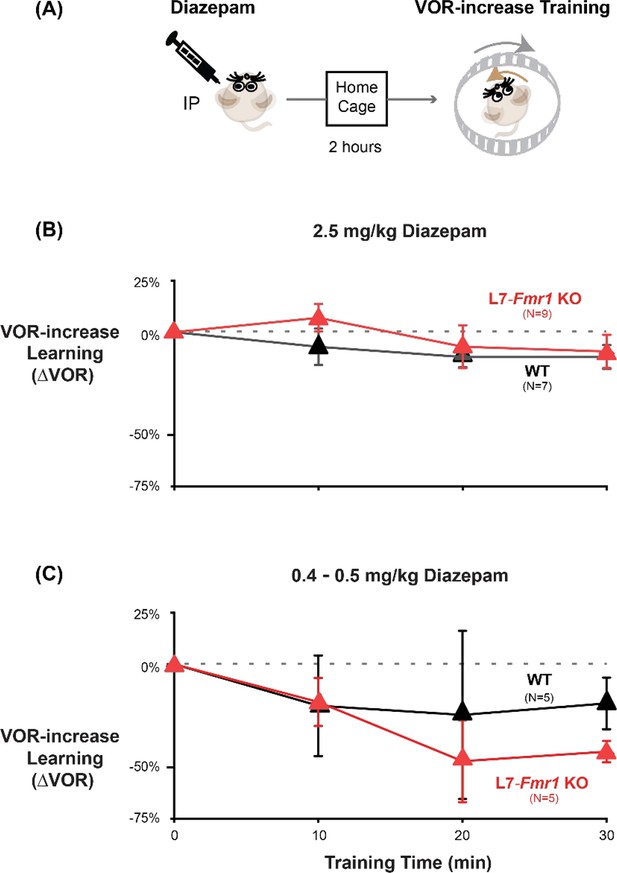
The acute effect of diazepam was inhibition of VOR-increase learning.
(A) Mice were given an intraperitoneal (IP) injection of diazepam (2.5 mg/kg or 0.4–0.5 mg/kg) and then returned to the home cage for 2 hr before VOR-increase training. When VOR-increase training was delivered two hours after IP injection of 2.5 mg/kg diazepam (B), 0.4–0.5 mg/kg diazepam (C), no learned increase in VOR amplitude was observed in L7-Fmr1 KO (red) or WT (black) mice.
Low frequency (0.5 Hz) VOR-increase learning impairment was not rescued by behavioral pre-training or diazepam pre-treatment.
(A) Low-frequency VOR-increase learning of L7-Fmr1 KO mice (red) and WT mice (black), without pre-training (left), after 0.5 Hz VOR-decrease pre-training (middle), and after 0.5 Hz Vestibular only pre-training (right). (B) Low frequency (0.5 Hz) VOR-increase learning 18–24 hr after IP injection of saline (left) or 0.5 mg/kg diazepam (right).
-
Figure 4—source data 1
Low frequency (0.5 Hz) VOR-increase learning impairment was not rescued by behavioral pre-training or diazepam pre-treatment.
- https://cdn.elifesciences.org/articles/92543/elife-92543-fig4-data1-v1.xlsx
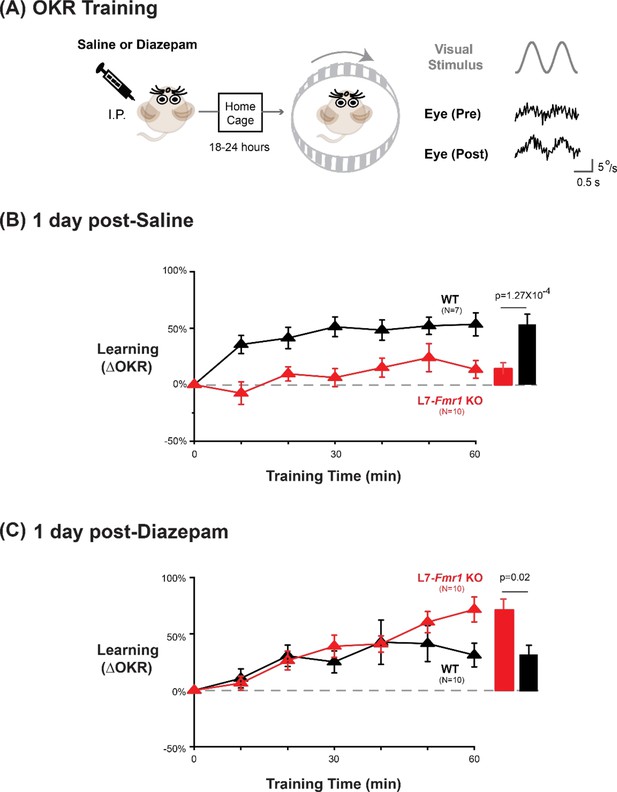
Diazepam pre-treatment rescued OKR learning impairment of L7-Fmr1 KO mice with enhanced associative LTD.
(A) Left, OKR adaptation was assessed 18–24 hr after a single injection of saline or diazepam. Middle OKR adaptation was induced by rotating a striped optokinetic drum about an earth-vertical axis centered on the head of the mouse with a 1 Hz sinusoidal velocity profile and peak velocity of ± 10°/s. Right, Example raw eye velocity responses (black) to the optokinetic visual stimulus (gray), measured at the beginning (Pre) and end (Post) of 60 min of OKR adaptation training. (B) Average learned change in the amplitude of the OKR relative to pre-training, after each 10 min OKR training block in the L7-Fmr1 KO (red) and WT mice (black), 1 day after saline injection. (C) Average learned change in the amplitude of the OKR relative to pre-training in L7-Fmr1 KO (red) and WT (black) mice that received diazepam (0.5 mg/kg) the previous day.
-
Figure 5—source data 1
Diazepam pre-treatment rescued OKR learning impairment of L7-Fmr1 KO mice with enhanced associative LTD.
- https://cdn.elifesciences.org/articles/92543/elife-92543-fig5-data1-v1.xlsx
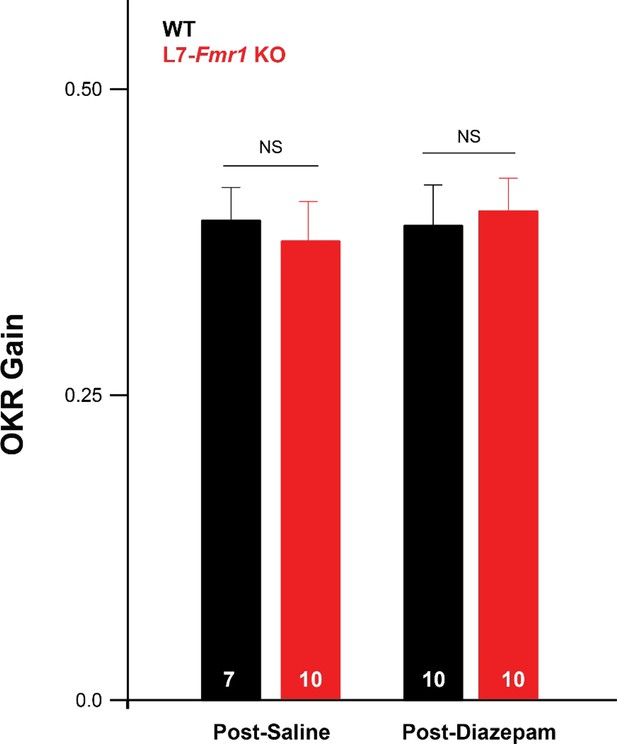
Baseline optokinetic reflex (OKR) performance normal in L7-Fmr1 KO mice and after diazepam pre-treatment.
The baseline OKR was measured during the first three minutes of OKR adaptation training in L7-Fmr1 KO (red) and WT (black) mice, 18–24 hr after an IP injection of saline or diazepam (0.5 mg/kg). There was no difference in the baseline OKR gain (ratio of eye velocity to optokinetic drum velocity) of L7-Fmr1 KO vs. WT mice (p=0.690 Pre) and no effect of diazepam pre-treatment on the baseline OKR performance (L7-Fmr1 KO, p=0.690, post-saline vs. post-diazepam; WT, p=0.55, post-saline vs. post-diazepam; Tukey).
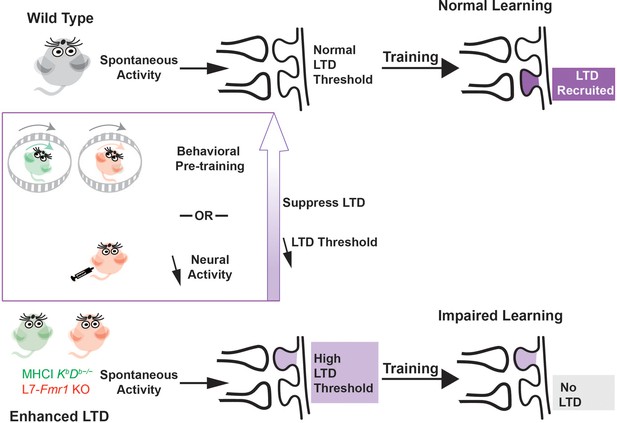
Metaplasticity hypothesis for how enhanced plasticity could impair learning.
Top, In naïve wild type mice, synapses have a normal threshold for associative LTD, and undergo LTD (dark violet) in response to training, thereby supporting normal learning. Bottom, In mice with enhanced LTD, such as L7-Fmr1 KO (pink) and MHCI KbDb−/− (green), the lower threshold for induction of LTD allows it to be aberrantly recruited by spontaneous activity in the circuit (light violet), thereby increasing the threshold for additional LTD induction. This prevents the recruitment of LTD during training at the synapses where it is needed to support learning (grey box), which impairs learning. Behavioral pre-training or drugs that reduce neural activity can suppress LTD induction and reset the threshold for LTD to normal (upward purple arrow), restoring the capacity for LTD-dependent learning.





