Insights into early animal evolution from the genome of the xenacoelomorph worm Xenoturbella bocki
Figures
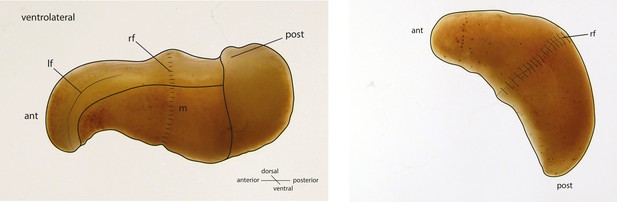
Schematic drawings of X. bocki showing the simple body organization of the marine vermiform animal.
ant, anterior; post, posterior; If, lateral furrow; rf, ring furrow; m, mouth opening.
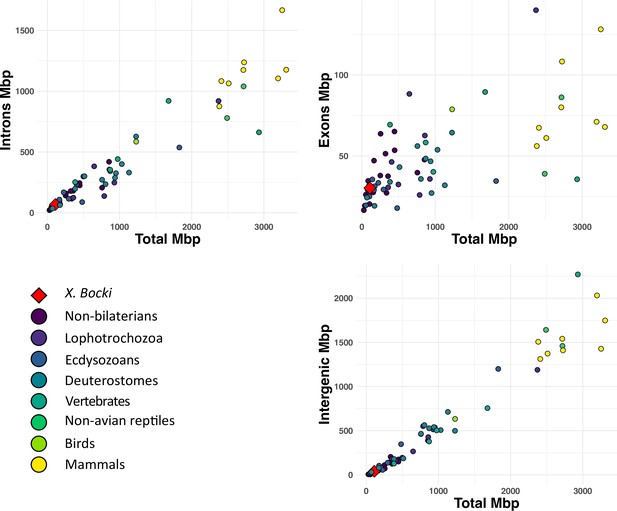
A comparison of total length of exons, intrans, and intergeneic space in the X. bocki genome with other metazoans (data from Francis and Wörheide, 2017).
X. bocki does not appear to be an outlier in any of these comparisons.
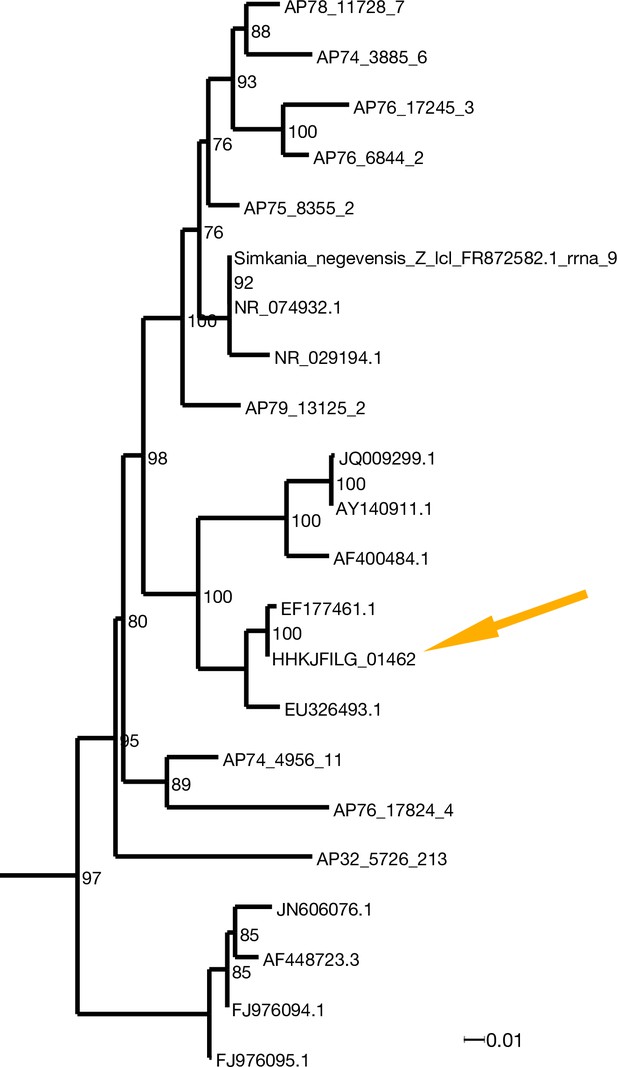
X. bocki harbors a marine Chlamydiae species as potential symbiont.
In the phylogenetic analysis of 16S rDNA (ML: GTR + F + R7; bootstrap values included) the bacteria in our X. bocki isolate (arrow) are sister lo a previous isolate from X. westbladi. X. westbladi is most likely a mis-identification of X. bocki.
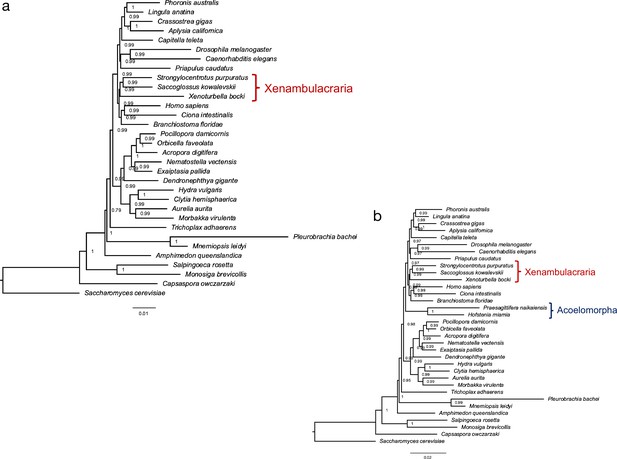
A phylogeny based on the presence and absence of genes calculated with OMA.
Both analysis (a) and (b) confirm Xenambulacraria, that is, Xenoturbellida in a group with Echinoderms and Hemichordates. Inclusion of the acoel flatworms places these as sister to all other Bilateria (b). This placement appears as an artifact due to the very fast evolution in this taxon, in particular as good evidence exists for uniting Xenoturbellida and Acoela (Philippe et al., 2019; Cannon et al., 2016; Rouse et al., 2016; Srivastava et al., 2014; Philippe et al., 2011; Bourlat et al., 2006; Ueki et al., 2019).
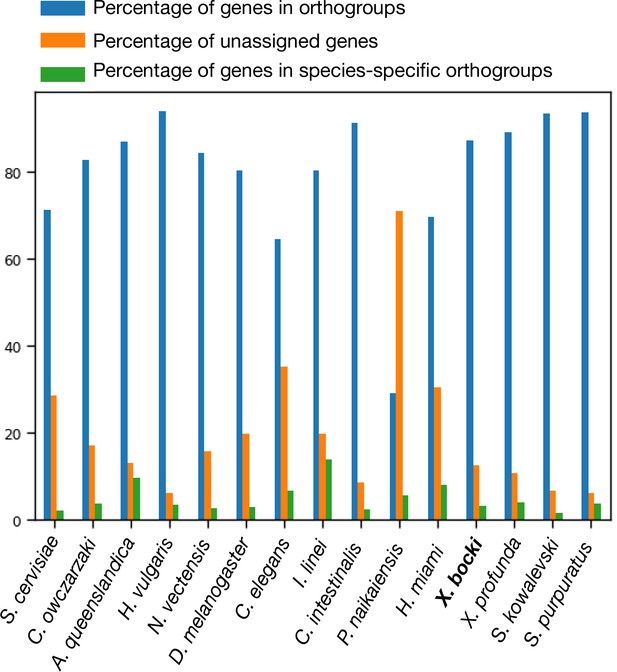
In our orthology screen, X. bocki shows similar percentages of genes in orthogroups, unassigned genes, and species-specific orthogroups as other well-annotated enomes.
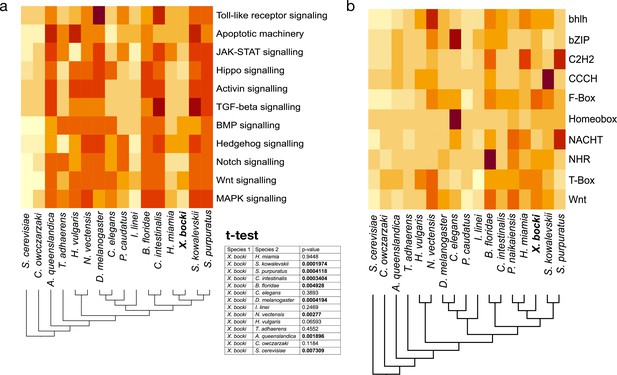
The heatmaps show a comparative measure of relative completeness of signaling pathways based on KEGG and assessed with GenomeMaple or abundance of genes in a given gene-family based on lnterProsScan annotations.
(a) Cell signaling pathways in X. bocki are functionally complete, but in comparison to other species contain less genes. The overall completeness is not significantly different to, for example, the nematode C. elegans (inset, t-test). (b) The number of family members per species in major gene families (based on Pfam domains), like transcription factors, fluctuates in evolution. The X. bocki genome does not appear to contain particularly less or more genes in any of the analyzed families. Due to the comparative nature of the assay, no ‘true’ scale can be given: darker colors indicate higher comparative completeness. Schematic cladograms are drawn by the authors.
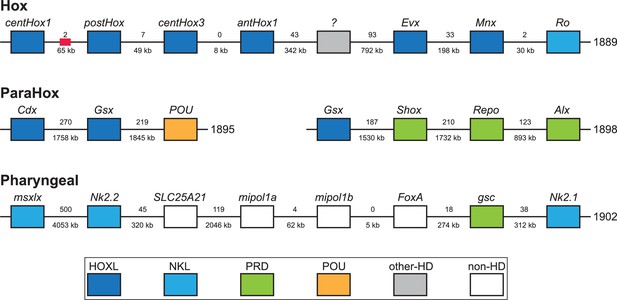
X. bocki has five HOX genes, which are located in relatively close proximity on one of our chromosome-size scaffolds.
Similar clusters exist for the ParaHox and ‘pharyngeal’ genes. Numbers between genes are distance (below) and number of genes between (below). Colors indicate gene families. Red box marks the position of a partial Hox gene. The ‘?’ gene has an unresolved homeodomain identity.
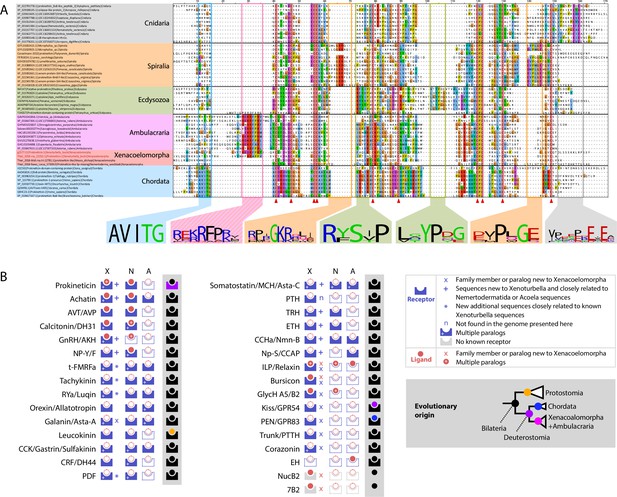
X.bocki genome contains genes for most bilaterian specific peptidergic system and a prokineticin gene containing a signature sequence shared with ambulacraria.
(a) Sequence alignment of Cnidarian Colipase-like protein, Ecdysozoan Astakine-like protein and Spiralian, Chordates and Xenacoelomorpha Prokineticin-like proteins show conserved cysteine positions (highlighted by red triangle), as well as clade specific signature sequences sequences among which a “K/R-RFP-K/R” sequence shared only by ambulacrarians and X. bocki. The signature previously reported for Ecdysozoa and chordata, as well as new signatures we found in Spiralia and Cnidaria is absent from ambulacrarians and X. bocki prokineticin ligand sequences. Sequences are available as Figure 8—source data 1; alignment files are available at https://doi.org/10.5281/zenodo.6962271. (b) Peptidergic systems found in Xenoturbella (X), Nemertodermatida (N) and Acoelomorpha (A). Novel findings are highlighted in the top right inset. Color of schemes and inset cladogram nodes on grey background depicts the evolutionary origin of peptidergic systems in accordance with our analysis: bilaterian, protostomian, chordate, xenacoelomorph + ambulacrarian last common ancestors respectively. 7B2, Neuroendocrine protein 7B2; AKH, adipokinetic hormone; Asta-A, Allatostatin-A; Asta-C, Allatostatin-C; AVP, arginine vasopressin; AVT, Arginine vasotocin; CCAP, crustacean cardioactive peptide; CCHa, CCHamide peptide; CCK, cholecystokinin; CRF, Corticotropin-releasing factor; DH31, diuretic hormone 31; DH44, diuretic hormone 44; EH, eclosion hormone; GlycH A5, Glycoprotein Hormone alpha5; GlycH B2, Glycoprotein Hormone beta2; GnRH, Gonadotropin Releasing Hormone; GPR54, G Protein-Coupled Receptor 54; GPR83, G Protein-Coupled Receptor 83; ILP, Insulin-like peptide; Kiss, Kisspeptine; MCH, melanin concentrating hormone; Nmn-B, Neuromedin B; Np-S, Neuropeptide S; NP-Y/F, Neuropeptide Y/F; NucB2, nucleobindin 2; PDF, Pigment-dispersing factor; PEN, neuroendocrine peptide PEN; PTTH, Prothoracicotropic hormone; RYa, RYamide peptide; t-FMRFa, trochozoan-FMRFamide peptide.
-
Figure 8—source data 1
Xenoturbella bocki neuropeptide sequences.
- https://cdn.elifesciences.org/articles/94948/elife-94948-fig8-data1-v2.docx
-
Figure 8—source data 2
Xenoturbella bocki neuropeptide receptor sequences.
- https://cdn.elifesciences.org/articles/94948/elife-94948-fig8-data2-v2.docx
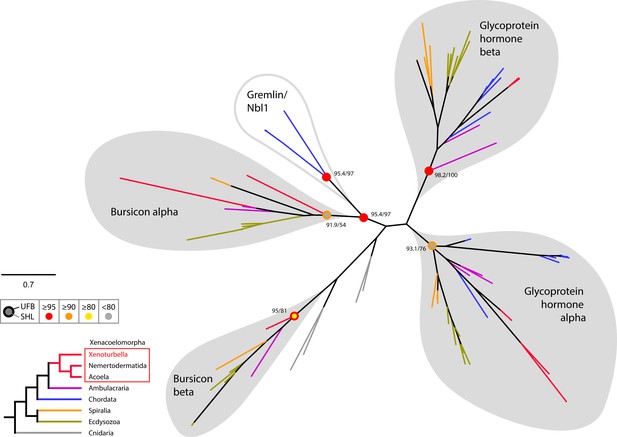
Radial tree representation of the phylogenetic analysis of bilaterian glycoprotein hormone and Bursicon.
Colored dots indicate support (UFB, 1000 ultrafast bootstrap replicates; SHL, 1000 SH-aLRT replicates) and follow the color code in the left inset. Scale bar unit for branch length is the number of substitutions per site. Branches are colored according to the phylogenetic position of the organism from which the sequence originates and follow the color code in the left inset. Nbl1, neuroblastoma suppressor of tumorigenicity 1. Sequences are available as Figure 8—source data 1; alignment and IQTREE tree files are available at https://doi.org/10.5281/zenodo.6962271.
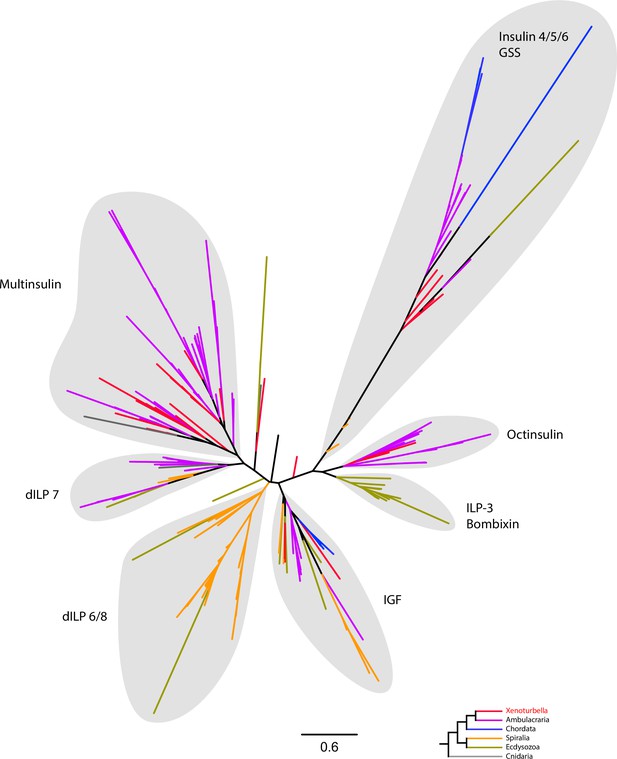
Radial tree representation of the sequence similarities analysis of bilaterian insulin-related peptides.
Tree is calculated from concatenated alignment of A and B chains. Scale bar unit for branch length is the number of substitutions per site. Branches are colored according to the phylogenetic position of the organism from which the sequence originates and follow the color code in the bottom inset. dILP, Drosophila insulin-like peptide; GSS, gonad-stimulating substance; ILP, insulin-like peptide; IGF, insulin-like growth factor. Full version of this tree is presented as Supplementary file 2. Sequences are available as Figure 8—source data 1; alignment and IQTREE tree files are available at https://doi.org/10.5281/zenodo.6962271.
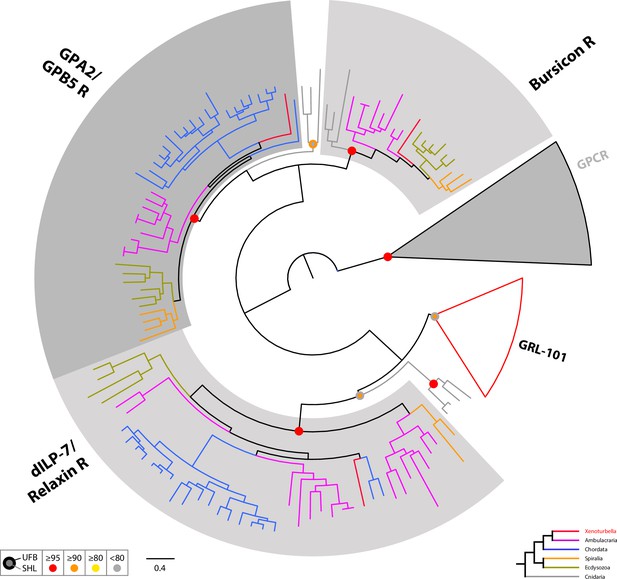
Circular tree representation of the phylogenetic analysis of bilaterian Leucine-rich repeat-containing G-protein coupled Receptors (Rhodopsin type G-protein coupled Receptors delta).
Colored dots indicate support (UFB, 1000 Ultrafast bootstrap replicates; SHL, 1000 SH-aLRT replicates) and follow the color code in the bottom inset. Scale bar unit for branch length is the number of substitutions per site. Branches are colored according to the phylogenetic position of the organism from which the sequence originates and follow the color code in the bottom inset. Collapsed group colored in red indicate that they contain at least one X. bocki sequence. GPA2, Glycoprotein Hormone alpha5; GPB5, Glycoprotein Hormone beta2; GPCR, G Protein-Coupled Receptor; GRL-101, G-protein coupled receptor GRL101. Full version of this tree is presented in Supplementary file 3. Sequences are available as Figure 8—source data 2; alignment and IQTREE tree files are available at https://doi.org/10.5281/zenodo.6962271.
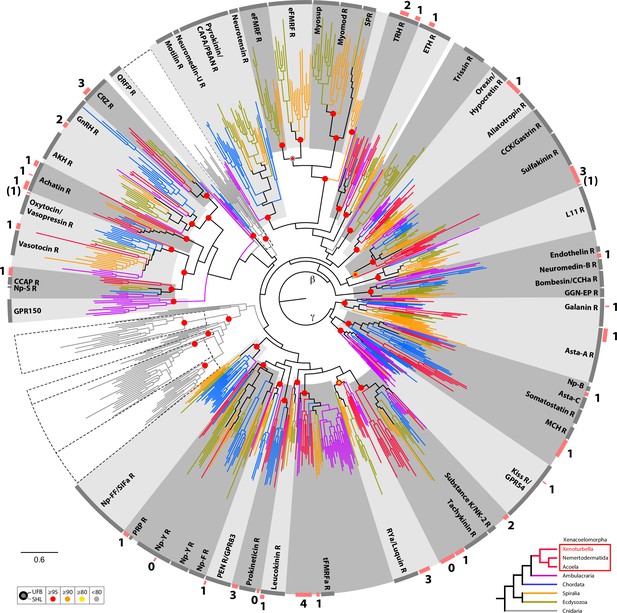
Circular tree representation of the phylogenetic analysis of bilaterian Rhodopsin type G-protein coupled Receptors beta and gamma.
Colored dots indicate support (UFB, 1000 ultrafast bootstrap replicates; SHL, 1000 SH-aLRT replicates) for main nodes and follow the color code in the bottom inset. Scale bar unit for branch length is the number of substitutions per site. Branches are colored according to the phylogenetic position of the organism from which the sequence originates and follow the color code in the bottom inset. Circular gray bars highlights names of groups of annotated sequences. Circular red bars indicate position of groups of Xenacoelomorpha sequences and associated number the number of X. bocki sequence(s) within these groups. AKH, adipokinetic hormone; Asta-A, Allatostatin-A; Asta-C, Allatostatin-C; CAPA, Cardio acceleratory peptide; CCAP, crustacean cardioactive peptide; CCHa, CCHamide peptide; CCK, cholecystokinin; CRZ, Corazonin; eFMRF, ecdysozoan-FMRFamide peptide; GGN-EP, GGN excitatory peptide; ETH, ecdysis triggering hormone; GnRH, Gonadotropin Releasing Hormone; GPR150, G Protein-Coupled Receptor 150; GPR54, G Protein-Coupled Receptor 54; GPR83, G Protein-Coupled Receptor 83; MCH, melanin concentrating hormone; Myomod, Myomodulin; NK-2, Neurokinin 2; Np-B/ W, Neuropeptide B/W; Np-FF, Neuropeptide FF; Np-F, Neuropeptide F; Np-S, Neuropeptide S; Np-Y, Neuropeptide Y; PBAN, pheromone biosynthesis activation neuropeptide; PEN, neuroendocrine peptide PEN; PRP, Prolactin releasing peptide; QRFP, Neuropeptide QRFP; RYa, RYamide peptide; SIFa, SIFamide peptide; SPR, Sex peptide receptor; tFMRFa, trochozoan-FMRFamide peptide; TRH, thyrotrophin-releasing hormone. Full version of this tree is presented in Supplementary file 4. Sequences are available as Figure 8—source data 2; alignment and IQTREE tree files are available at https://doi.org/10.5281/zenodo.6962271.
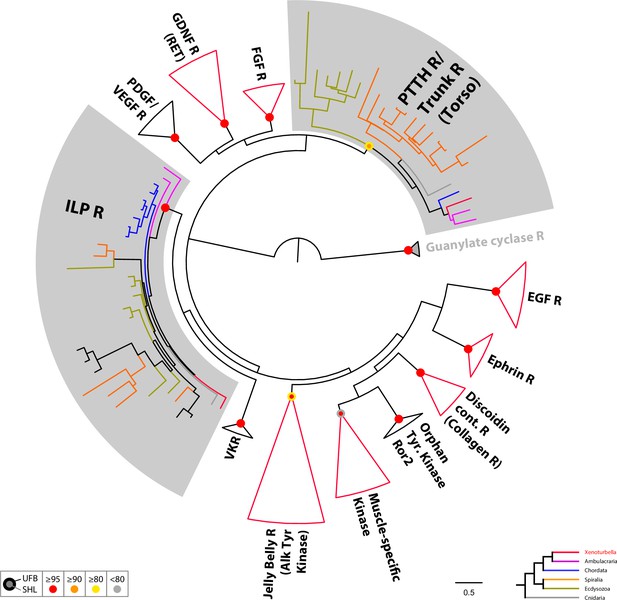
Circular tree representation of the phylogenetic analysis of bilaterian Tyrosine kinase Receptors.
Colored dots indicate support (UFB, 1000 ultrafast bootstrap replicates; SHL, 1000 SH-aLRT replicates) and follow the color code in the bottom inset. Scale bar unit for branch length is the number of substitutions per site. Branches are colored according to the phylogenetic position of the organism from which the sequence originates and follow the color code in the bottom inset. Collapsed group colored in red indicate that they contain at least one X. bocki sequence. EGF, Epidermal Growth Factor; Discoidin cont. R, discoidin domain-containing receptor; Orphan Tyr. Kinase Ror2, receptor tyrosine kinase-like orphan receptor 2; VKR, Venus kinase Receptor; ILP, Insulin-like peptide; PDGF, Platelet-derived growth factor; VEGF, Vascular endothelial growth factor; GDNF, Glial cell line-derived neurotrophic factor; FGF, fibroblast growth factor; PTTH, Prothoracicotropic hormone. Full version of this tree is presented in Supplementary file 5. Sequences are available as Figure 8—source data 2; alignment and IQTREE tree files are available at https://doi.org/10.5281/zenodo.6962271.
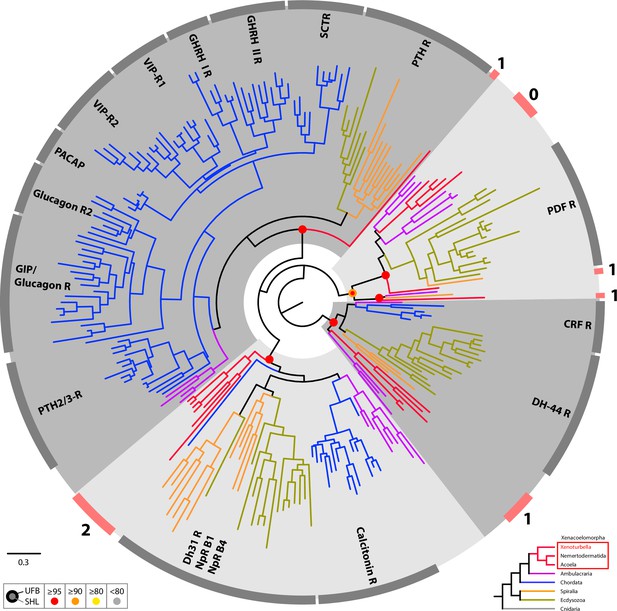
Circular tree representation of the phylogenetic analysis of bilaterian Secretin type G-protein coupled Receptors.
Colored dots indicate support (UFB, 1000 ultrafast bootstrap replicates; SHL, 1000 SH-aLRT replicates) for main nodes and follow the color code in the bottom inset. Scale bar unit for branch length is the number of substitutions per site. Branches are colored according to the phylogenetic position of the organism from which the sequence originates and follow the color code in the bottom inset. Circular gray bars highlights names of groups of annotated sequences. Circular red bars indicate position of groups of Xenacoelomorpha sequences and associated number the number of X. bocki sequence(s) within these groups. DH31, diuretic hormone 31; Np-R B1, Neuropeptide receptor B3; Np-R B4, Neuropeptide receptor B1; PDF, Pigment-dispersing factor; CRF, Corticotropin-releasing factor; DH-44, diuretic hormone 44; PTH2/3-R, Parathyroid hormone receptor2/3; GIP, Gastric inhibitory polypeptide; PACAP, Pituitary adenylate cyclase-activating polypeptide; VIP-R, Vasoactive intestinal polypeptide receptor; GHRH, Growth hormone-releasing hormone; PTH, Parathyroid hormone receptor; SCTR, Secretin Receptor. Full version of this tree is presented in Supplementary file 6. Sequences are available as Figure 8—source data 2; alignment and IQTREE tree files are available at https://doi.org/10.5281/zenodo.6962271.
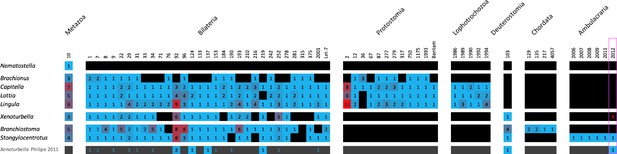
The rev sed microRNA complement of X. bocki has a near-complete set of metazoan, bilaterian, and deuterostome families and genes.
Presence (color) and absence (black) of microRNA families (column), paralog numbers (values and heatmap coloring) organized in node-specific blocks in a range of representative protostome and deuterostome species compared with Xenoturbella (species from MirGeneDB 2.1; Fromm et al., 2022). The bottom row depicts 2011 complement by Philippe et al., 2011 (blue numbers on black depict detected miRNA reads, but lack of genomic evidence). Red ‘x’ in the pink box highlights the lack of evidence for an Ambulacraria-specific microRNA in X. bocki.
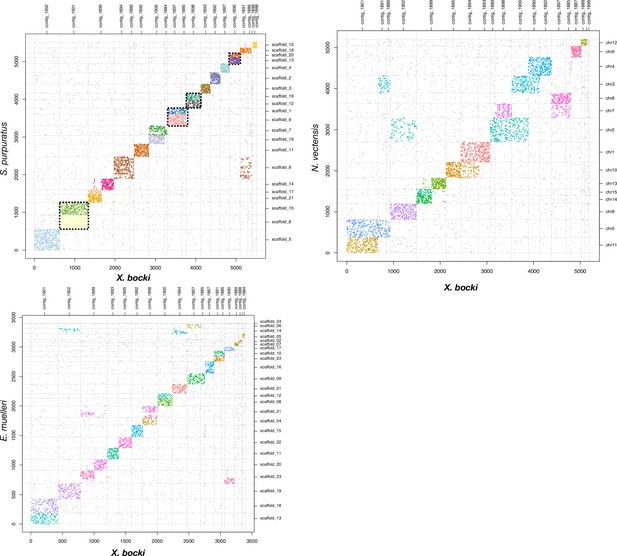
A comparison of scaffolds in the X. bocki genome with other Metazoa.
17 of the 18 large scaffolds in the X. bocki genome are linked via synteny to distinct chromosomal scaffolds in these species.
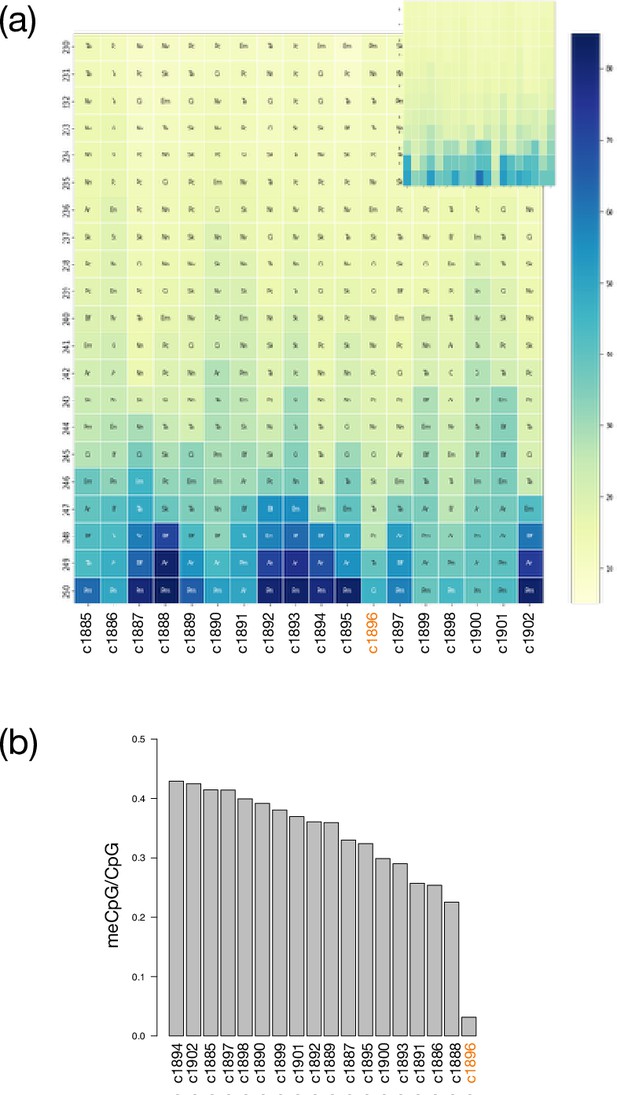
Conservation of metazoan synteny and methylation in X. bocki.
(a) A summary plot of synteny between major scaffolds in the X. bocki genome assembly and early branching highly contiguous metazoan genome assemblies: Euphydatia muelleri, Trichoplax adhearens, Branchiostoma floridae, Saccoglossus kowalevskii, Ciona intestinalis, Nematostella vectensis, Asteria rubens, Pecten maximus, Nemopilema nomurai, and Carcinoscorpius rotundicauda. All but one of the chromosome-sized scaffolds in our assembly have at least one syntenic match in the each of the other species (see the main text for one-to-one plots with key species and a description of the aberrant scaffold). We performed the same analysis with amphioxus as the focal species as a proof of principle (inset). (b) Analysis of methylation on the largest scaffold in the X. bocki genome assembly. One scaffold with a deviant gene age and synteny structure (see the main text) also stands out in terms of methylation. A detailed analysis of methylation patterns across the genome and classes of genes will be published separately.
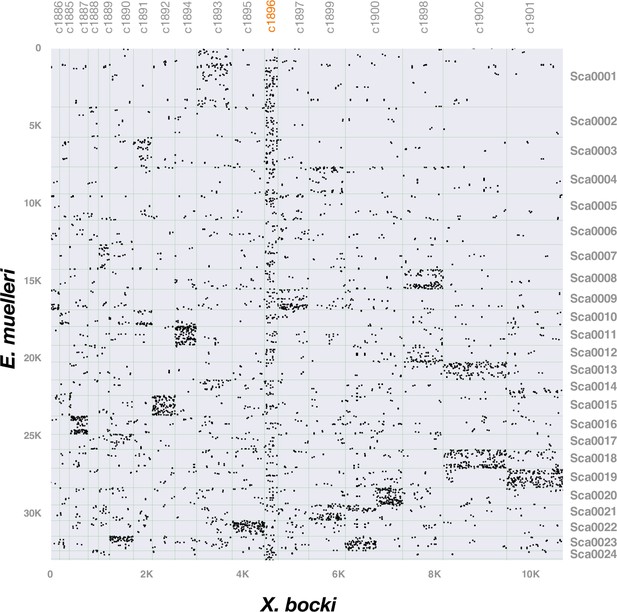
Intergenomic comparison of X. bocki and E. muelleri highlighting synteny connections between the aberrant scaffold c1896 and scaffolds across the sponge genome.
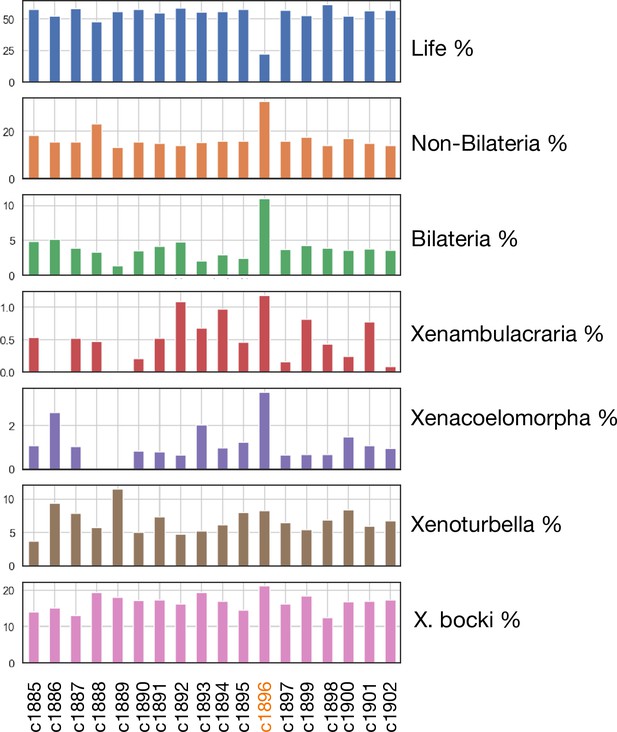
Phylostratigraphic age distribution of genes on all major scaffolds in the X. bocki genome.
One scaffold (c1896), which showed no synteny to a distinct chromosomal scaffold in the other metazoan species, also had a divergent gene age structure in comparison to other X. bocki scaffolds.
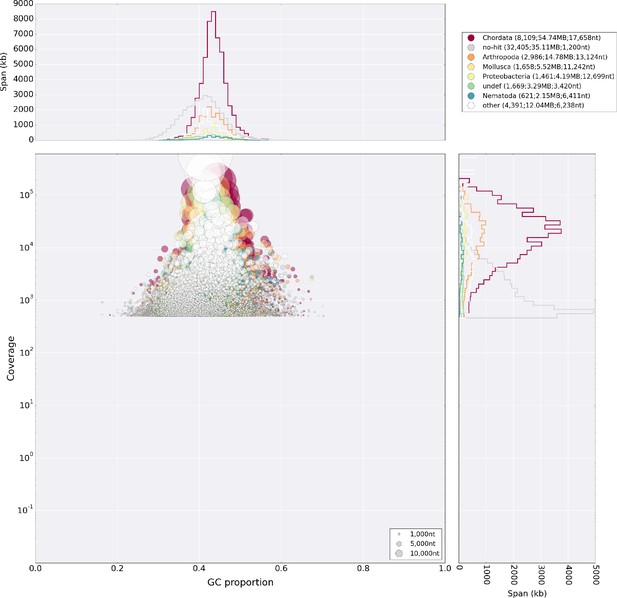
Blobplot analysis of the primary lllumina genome assembly.
The assembly shows no major microorganismal contamination, apart from the Chlamydia and Gammaproteobacteria described in the main text. The diamond tool was used to blast against the UniProt database for this analysis.
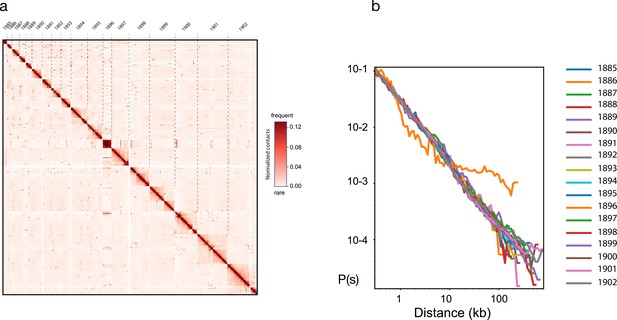
Hi-C based genome scaffolding with instaGRAAL.
(a) Contact frequency map of the largest 18 scaffolds and (b) distribution of contact frequency as a function of distance (distance law).
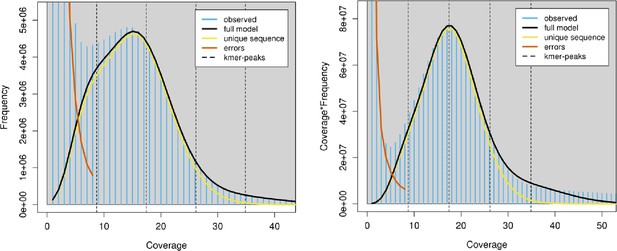
Kmer profile of the X. bocki Illumina WGS reads obtained with GenomeScope2 (Ranallo-Benavidez et al., 2020).
Linear plot and transformed linear plots are shown. As per the description at http://qb.cshl.edu/genomescope/, we used 21mers counted with jellyfish (Marçais and Kingsford, 2011). GenomeScope genome property estimates and measures were len: 222,242,800 bp, uniq: 31.7%, aa: 99.1%, ab: 0.929%, kcov: 8.72, err: 0.665%, dup: 0.527, k: 21, p:2, model fit min: 34.6%, model fit max: 96.3.
Tables
Improvement of assembly and scaffolding metrics.
| Assembly step | # seqs | # reals | # Ns | Max length | N50 |
|---|---|---|---|---|---|
| Redundans contigs | 37,880 | 113,212,556 | 38,3327 | 206,709 | 8544 |
| Redundans scaffolds | 24,538 | 117,405,089 | 3,021,351 | 952,321 | 52,073 |
| Pre instaGRAAL | 23,094 | 117,396,873 | 3,534,582 | 960,978 | 61,989 |
| Final scaffolds | 27,939 | 107,712,917 | 3,328,069 | 8,757,424 | 2,730,651 |
-
Assessed with the jvci toolbox: https://github.com/tanghaibao/jcvi (Tang, 2010).
Additional files
-
Supplementary file 1
Excel table with data sources for OrthoFinder analysis.
- https://cdn.elifesciences.org/articles/94948/elife-94948-supp1-v2.xlsx
-
Supplementary file 2
Full tree representation of the sequence similarities analysis of bilaterian insulin-related peptides.
Tree is calculated from concatenated alignment of A and B chains. Numbers represent support for nodes calculated using 1000 ultrafast bootstrap replications and 1000 SH-aLRT replicates, respectively. Scale bar unit for branch length is the number of substitutions per site. Branches are colored according to the phylogenetic position of the organism from which the sequence originates: red, Xenoturbella; pink, Ambulacraria; blue, Chordata; orange, Ecdysozoa; green, Ecdysozoa; gray, Cnidaria. dILP, Drosophila insulin-like peptide; GSS, gonad-stimulating substance; ILP, insulin-like peptide; IGF, insulin-like growth factor. Radial version of this tree is presented in Figure 8—figure supplement 2. Sequences are available as Figure 8—source data 1; alignment and IQTREE tree files are available at https://doi.org/10.5281/zenodo.6962271.
- https://cdn.elifesciences.org/articles/94948/elife-94948-supp2-v2.pdf
-
Supplementary file 3
Full tree representation of the phylogenetic analysis of bilaterian Leucine-rich repeat-containing G-protein coupled Receptors (Rhodopsin type G-protein coupled Receptors delta).
Numbers represent support for nodes calculated using 1000 Ultrafast bootstrap replications and 1000 SH-aLRT replicates respectively. Scale bar unit for branch length is the number of substitutions per site. Branches are colored according to the phylogenetic position of the organism from which the sequence originates: red, Xenoturbella; pink, Ambulacraria; blue, Chordata; orange, Ecdysozoa; green, Ecdysozoa; gray, Cnidaria. Collapsed group colored in red indicate that they contain at least one X. bocki sequence. GPA2, Glycoprotein Hormone alpha5; GPB5, Glycoprotein Hormone beta2; GPCR, G Protein-Coupled Receptor; GRL-101, G-protein coupled receptor GRL101. Circular version of this tree is presented in Figure 8—figure supplement 3. Sequences are available as Figure 8—source data 2; alignment and IQTREE tree files are available at https://doi.org/10.5281/zenodo.6962271.
- https://cdn.elifesciences.org/articles/94948/elife-94948-supp3-v2.pdf
-
Supplementary file 4
Full tree representation of the phylogenetic analysis of bilaterian Rhodopsin type G-protein coupled Receptors beta and gamma.
Numbers represent support for nodes calculated using 1000 ultrafast bootstrap replications and 1000 SH-aLRT replicates respectively. Scale bar unit for branch length is the number of substitutions per site. Branches are colored according to the phylogenetic position of the organism from which the sequence originates: red, Xenoturbella; pink, Ambulacraria; blue, Chordata; orange, Ecdysozoa; green, Ecdysozoa; gray, Cnidaria. White boxes with associated name highlight groups of annotated sequences. AKH, adipokinetic hormone; Asta-A, Allatostatin-A; Asta-C, Allatostatin-C; CAPA, Cardio acceleratory peptide; CCAP, crustacean cardioactive peptide; CCHa, CCHamide peptide; CCK, cholecystokinin; CRZ, Corazonin; eFMRF, ecdysozoan-FMRFamide peptide; GGN-EP, GGN excitatory peptide; ETH, ecdysis triggering hormone; GnRH, Gonadotropin Releasing Hormone; GPR150, G Protein-Coupled Receptor 150; GPR54, G Protein-Coupled Receptor 54; GPR83, G Protein-Coupled Receptor 83; MCH, melanin concentrating hormone; NK-2, Neurokinin 2; Np-B/W, Neuropeptide B/W; Np-FF, Neuropeptide FF; Np-F, Neuropeptide F; Np-S, Neuropeptide S; Np-Y, Neuropeptide Y; PBAN, pheromone biosynthesis activation neuropeptide; PEN, neuroendocrine peptide PEN; PRP, Prolactin releasing peptide; QRFP, Neuropeptide QRFP; RYa, RYamide peptide; SIFa, SIFamide peptide; SPR, Sex peptide receptor; tFMRFa, trochozoan-FMRFamide peptide; TRH, thyrotrophin-releasing hormone. Circular version of this tree is presented in Figure 8—figure supplement 4. Sequences are available as Figure 8—source data 2; alignment and IQTREE tree files are available at https://doi.org/10.5281/zenodo.6962271.
- https://cdn.elifesciences.org/articles/94948/elife-94948-supp4-v2.pdf
-
Supplementary file 5
Full tree representation of the phylogenetic analysis of bilaterian Tyrosine kinase Receptors.
Numbers represent support for nodes calculated using 1000 ultrafast bootstrap replications and 1000 SH-aLRT replicates respectively. Scale bar unit for branch length is the number of substitutions per site. Branches are colored according to the phylogenetic position of the organism from which the sequence originates: red, Xenoturbella; pink, Ambulacraria; blue, Chordata; orange, Ecdysozoa; green, Ecdysozoa; gray, Cnidaria. Collapsed group colored in red indicate that they contain at least one X. bocki sequence. EGF, Epidermal Growth Factor;Discoidin cont. R, discoidin domain-containing receptor; Orphan Tyr. Kinase Ror2, receptor tyrosine kinase-like orphan receptor 2; VKR, Venus kinase Receptor; ILP, Insulin-like peptide; PDGF, Platelet-derived growth factor; VEGF, Vascular endothelial growth factor; GDNF, Glial cell line-derived neurotrophic factor; FGF, fibroblast growth factor; PTTH, Prothoracicotropic hormone. Circular version of this tree is presented in Figure 8—figure supplement 5. Sequences are available as Figure 8—source data 2; alignment and IQTREE tree files are available at https://doi.org/10.5281/zenodo.6962271.
- https://cdn.elifesciences.org/articles/94948/elife-94948-supp5-v2.pdf
-
Supplementary file 6
Full tree representation of the phylogenetic analysis of bilaterian Secretin type G-protein coupled Receptors.
Numbers represent support for nodes calculated using 1000 ultrafast bootstrap replications and 1000 SH-aLRT replicates respectively. Scale bar unit for branch length is the number of substitutions per site. Branches are colored according to the phylogenetic position of the organism from which the sequence originates: red, Xenoturbella; pink, Ambulacraria; blue, Chordata; orange, Ecdysozoa; green, Ecdysozoa; gray, Cnidaria. White boxes with associated name highlight groups of annotated sequences. DH31,diuretic hormone 31; Np-RB1, Neuropeptide receptor B3; Np-RB4, Neuropeptide receptor B1; PDF, Pigment-dispersing factor; CRF, Corticotropin-releasingfactor; DH-44,diuretic hormone 44; PTH2/3-R,Parathyroid hormonereceptor2/3; GIP, Gastric inhibitory polypeptide; PACAP, Pituitary adenylate cyclase-activating polypeptide;VIP-R,Vasoactive intestinal polypeptide receptor; GHRH, Growth hormone-releasing hormone; PTH, Parathyroid hormone receptor; SCTR, Secretin Receptor. Circular version of this tree is presented in Figure 8—figure supplement 6. Sequences are available as Figure 8—source data 2; alignment and IQTREE tree files are available at https://doi.org/10.5281/zenodo.6962271.
- https://cdn.elifesciences.org/articles/94948/elife-94948-supp6-v2.pdf
-
Supplementary file 7
Sequence alignment of bilaterian 7B2 neuropeptides.
The alignment highlights the presence in all sequence of aconserved ‘PPNPCP’ motif. X. bocki sequence is highlighted by a red dashed line. Sequences are available as Figure 8—source data 1; alignment is available at https://doi.org/10.5281/zenodo.6962271.
- https://cdn.elifesciences.org/articles/94948/elife-94948-supp7-v2.pdf
-
Supplementary file 8
Sequence alignment of Xenacoelomorpha LRIGamide neuropeptides.
X. bocki sequence is highlighted by a reddashed line. Sequences are available as Figure 8—source data 1; alignment is available at https://doi.org/10.5281/zenodo.6962271.
- https://cdn.elifesciences.org/articles/94948/elife-94948-supp8-v2.pdf
-
Supplementary file 9
Sequence alignment of bilaterian Nucleobindin2/Nesfatin neuropeptides.
X. bocki sequence is highlighted by a reddashed line. Sequences are available as Figure 8—source data 1; alignment is available at https://doi.org/10.5281/zenodo.6962271.
- https://cdn.elifesciences.org/articles/94948/elife-94948-supp9-v2.pdf
-
Supplementary file 10
Sequence alignment of Ambulacrarian octinsulin with a potential X. bocki homolog sequence.
Red trianglehighlights the conserved cysteine positions. X. bocki sequence is highlighted by a red dashed line. Sequences are available as Figure 8—source data 1; alignment is available at https://doi.org/10.5281/zenodo.6962271.
- https://cdn.elifesciences.org/articles/94948/elife-94948-supp10-v2.pdf
-
MDAR checklist
- https://cdn.elifesciences.org/articles/94948/elife-94948-mdarchecklist1-v2.docx





