Author Response
The following is the authors’ response to the original reviews.
Public Reviews:
Reviewer #1 (Public Review):
The association of vitamin D supplementation in reducing Asthma risk is well studied, although the mechanistic basis for this remains unanswered. In the presented study, Kilic and co-authors aim to dissect the pathway of Vitamin D mediated amelioration of allergic airway inflammation. They use initial leads from bioinformatic approaches, which they then associate with results from a clinical trial (VDAART) and then validate them using experimental approaches in murine models. The authors identify a role of VDR in inducing the expression of the key regulator Ikzf3, which possibly suppresses the IL-2/STAT5 axis, consequently blunting the Th2 response and mitigating allergic airway inflammation.
Strengths:
The major strength of the paper lies in its interdisciplinary approach, right from hypothesis generation, and linkage with clinical data, as well as in the use of extensive ex vivo experiments and in vivo approaches using knock-out mice.
The study presents some interesting findings including an inducible baseline absence/minimal expression of VDR in lymphocytes, which could have physiological implications and needs to be explored in future studies.
Weaknesses:
The core message of the study relies on the role of vitamin D and its receptor in suppressing the Th2 response. However, there is scope for further dissection of relevant pathophysiological parameters in the in vivo experiments, which would enable stronger translation to allergic airway diseases like Asthma.
To a large extent, the authors have been successful in validating their results, although a few inferences could be reinforced with additional techniques, or emphasised in the discussion section (possibly utilising the ideas and speculative section offered by the journal).
The study inferences also need to be read in the context of the different sub-phenotypes and endotypes of Asthma, where the Th2 response may not be predominant. Moreover, the authors have referenced vitamin D doses for the murine models from the VDAART trials and performed the experiments in the second generation of animals. While this is appreciated, the risk of hypervitaminosis-D cannot be ignored, in view of its lipid solubility. Possibly comparison and justification of the doses used in murine experiments from previous literature, as well as the incorporation of an emphasised discussion about the side effects and toxicity of Vitamin D, is an important aspect to consider.
In no way do the above considerations undermine the importance of this elegant study which justifies trials for vitamin D supplementation and its effects on Asthma. The work possesses tremendous potential.
We thank the reviewer for their careful assessment of our paper and helpful suggestions. Please find the point-by-point responses to the reviewer recommendations below.
Reviewer #2 (Public Review):
Summary:
This study seeks to advance our knowledge of how vitamin D may be protective in allergic airway disease in both adult and neonatal mouse models. The rationale and starting point are important human clinical, genetic/bioinformatic data, with a proposed role for vitamin D regulation of 2 human chromosomal loci (Chr17q12-21.1 and Chr17q21.2) linked to the risk of immune-mediated/inflammatory disease. The authors have made significant contributions to this work specifically in airway disease/asthma. They link these data to propose a role for vitamin D in regulating IL-2 in Th2 cells implicating genes associated with these loci in this process.
Strengths:
Here the authors draw together evidence from multiple lines of investigation to propose that amongst murine CD4+ T cell populations, Th2 cells express high levels of VDR, and that vitamin D regulates many of the genes on the chromosomal loci identified to be of interest, in these cells. The bottom line is the proposal that vitamin D, via Ikfz3/Aiolos, suppresses IL-2 signalling and reduces IL-2 signalling in Th2 cells. This is a novel concept and whilst the availability of IL-2 and the control of IL-2 signalling is generally thought to play a role in the capacity of vitamin D to modulate both effector and especially regulatory T cell populations, this study provides new data.
Weaknesses:
Overall, this is a highly complicated paper with numerous strands of investigation, methodologies etc. It is not "easy" reading to follow the logic between each series of experiments and also frequently fine detail of many of the experimental systems used (too numerous to list), which will likely frustrate immunologists interested in this. There is already extensive scientific literature on many aspects of the work presented, much of which is not acknowledged and largely ignored. For example, reports on the effects of vitamin D on Th2 cells are highly contradictory, especially in vitro, even though most studies agree that in vivo effects are largely protective. Similarly, other reports on adult and neonatal models of vitamin D and modulation of allergic airway disease are not referenced. In summary, the data presentation is unwieldy, with numerous supplementary additions, which makes the data difficult to evaluate and the central message lost. Whilst there are novel data of interest to the vitamin D and wider community, this manuscript would benefit from editing to make it much more readily accessible to the reader.
Wider impact: Strategies to target the IL-2 pathway have long been considered and there is a wealth of knowledge here in autoimmune disease, transplantation, GvHD etc - with some great messages pertinent to the current study. This includes the use of IL-2, including low dose IL-2 to boost Treg but not effector T cell populations, to engineered molecules to target IL-2/IL-2R.
We thank the reviewer for their careful assessment of our paper and helpful suggestions. Please find the point-by-point responses to the reviewer recommendations below. In addition, we have revisited the Introduction and Discussion, added additional subsection headings, and provided additional schematics to make the general flow of the paper more accessible to a wider audience.
Reviewer #1 (Recommendations For The Authors):
There are certain aspects of the manuscript which could be revisited in order to provide more clarity to the reader. Some of these are:
- In vivo experiments : The major inference and its impact is derived from the effect of VDR on Ikzf3 expression, and consequently on the Th2 response. While the study employs both in vivo and ex vivo approaches to validate this claim, pathophysiological aspects could have been explored in more detail, by using cytokine panels, possibly techniques to measure airway resistance, as well as by reducing the variations in the sample sizes used in different groups. Similarly, certain inferences from ex vivo studies may be important to demonstrate in the in vivo setting as well. A justification for the incorporation of both Balb/c and C57 Bl6 mice for the experiments could also be incorporated in the manuscript.
- Certain sections, especially those connecting VDR, Ikzf1/3 and IL2/STAT axis seem associative. This is indicated by Figure 5 H as well, where the effects of calcitriol administration in KO cells indicate additional pathways at play, possibly through indirect effects. The use of additional techniques like ChIP, co-IP and establishing STAT induction/activation would probably strengthen the findings, alternatively, a clear distinction between the speculative and the definitive results could be made in the discussion section, as the journal encourages. Similar considerations could be made for VDR and Ikzf3.
- Role of other cells :
a. While the investigators have explored the phenotype on other cell types like Th1 and Treg, at places there remains a lacuna. For instance, the absence of neutrophil fractions from the DLC-BAL, as well inconsistencies in the groups selected for comparison. For eg. in Figure 3 Supplementary Figure 2, the figure suggests IL13 expression in CD4+ cells, yet the text reads incubated Th2 cells. This could be made more lucid.
b. In Figure 3 Supplementary Figure 1 there is a trend towards an increase in IL-10 levels, whereas in Supplementary Figure 2 there is a drop in the IL13 level in the VDR KO group, which has not been explained.
c. While 17q loci form the predominant loci associated with Asthma, other loci important in Asthma on chromosomes 2,6,9, 22 could be discussed in the manuscript as well, even if they can't be explored in depth.
- Quantification of histology and confocal images could provide an objective assessment to the readers. Possibly incorporation of co-localisation panels for the IF images showing membrane/cytoplasmic/ nuclear localisation of the VDR under various conditions.
- Structure of the manuscript: At places the manuscript has a disrupted flow, as well as mislabelled figures (Figure 2SF1B is 1C, Fig 2c is 2b in the results, ). Flow gates can be arranged sequentially and consistent labelling of the gates and axis would ease interpretation. In some places sample sizes mentioned do not match the dot graphs in the figures (figure 3K-L). In the same figure and others (Figure 5 Supplementary Figure 2), a comparison of all groups would be beneficial. A restructuring of the results and corrections, could assist the reader. Also, a visualization of the VDAART analysis in the main figures, corroborating with the results sections would do justice to the interesting approach and findings. The clearances and approvals for the study also need to be incorporated into the manuscript. If possible, the incorporation of a schematic showing the proposed pathway for VDR-induced Ikzf3 and subsequent suppression of the genes present on Chr 17 loci to mitigate allergic airway inflammation would help.
Reviewer #2 (Recommendations For The Authors):
A few specific points: A number of immune concepts are studied without reference to the broader literature and the data presented data on occasion counter these earlier findings. Examples of this include:
- Vitamin D can both enhance and inhibit IL-13 synthesis, demonstrated both in vitro and ex vivo, and these effects are clearly context-specific. I am not questioning the validity of the present experimental findings in this specific experimental model), but the experimental context - the problem is that this is not discussed.
- Short-term bulk Th2 cultures are used with no indication of their enrichment for lineage-specific markers or cytokine - their conclusions might be enhanced by this. Data on genes/markers of interest could be further enhanced by showing FACS plots of co-expression e.g. Th2 genes e.g. IL-13/GATA3 with these other markers.
- Are human Th2 enriched for VDR, since the backdrop to this study is human clinical and genetic data? For a study that has based its rationale on human clinical/genetic studies it would be great to confirm these findings in human Th2 cells.
- The Discussion might comment on some of these wider issues.
- Minor typos throughout, including in figure legends
Reviewer #1
- The study inferences also need to be read in the context of the different sub-phenotypes and endotypes of Asthma, where the Th2 response may not be predominant.
We agree that asthma has many sub-phenotypes and endotypes and that the Th2 response may not be predominant in all of them, but we focus here on the origins of the disease in the first few years of life and the genetic and molecular mechanisms associate with disease onset where the Th2 response is important.
- Moreover, the authors have referenced vitamin D doses for the murine models from the VDAART trials and performed the experiments in the second generation of animals. While this is appreciated, the risk of hypervitaminosis-D cannot be ignored, in view of its lipid solubility. Possibly comparison and justification of the doses used in murine experiments from previous literature, as well as the incorporation of an emphasized discussion about the side effects and toxicity of Vitamin D, is an important aspect to consider.
We appreciate this comment from the reviewers allowing us to review vitamin D toxicity in more detail. Given the length of this review we did not include this in the manuscript discussion but provide it here.
Vitamin D supplementation in humans is debated due to possibility of intoxication from overdose. Vitamin D intoxication is a rare medical condition associated with hypercalcemia, hyperphosphatemia, and suppressed parathyroid hormone level and is typically seen in patients who are receiving very high doses of vitamin D, ranging from 50,000 to 1 million IU/d for several months to years 1,2. Intoxication observed at lower doses might be attributable to rare genetic disorders 1. By far the bigger problem in humans is vitamin D deficiency; this is especially true in pregnant women where dosage requirements are high due to the needs of the fetus. It is estimated that virtually all pregnant women are vitamin D insufficient or deficient 3. VDAART has shown that vitamin D in a dose of 4400 IC given to pregnant women can prevent asthma in their offspring. There were no adverse side effects in the mother or the infant from this dose 4.
In rodents, a few studies have reported vitamin D intoxication with very high vitamin D doses 5(PMID: 23405058: 50.000 IU/kg 120d -> toxicity in females). In contrast there are several studies using 2-2.5 times higher doses of vitamin D than we use here, that do not report adverse events in mouse models of disease 6,7. Our doses of vitamin D are identical to those used in VDAART and are lower than those used in any of these other rodent studies. In addition, while we did not specifically assess specific signs of vitamin D intoxication, we can exclude any impact on animal well-being, health, reproduction, and behavior throughout the study.
- The major inference and its impact are derived from the effect of VDR on Ikzf3 expression, and consequently on the Th2 response. While the study employs both in vivo and ex vivo approaches to validate this claim, pathophysiological aspects could have been explored in more detail, by using cytokine panels, possibly techniques to measure airway resistance, as well as by reducing the variations in the sample sizes used in different groups.
We have added the following sentence to the discussion: “Additional cytokine measurements in the mice as well as measurement of airway resistance would have added to the pathophysiological data linking IKFZ3 expression to TH2 response.”
- Similarly, certain inferences from ex vivo studies may be important to demonstrate in the in vivo setting as well. A justification for the incorporation of both Balb/c and C57 Bl6 mice for the experiments could also be incorporated in the manuscript.
We agree with the reviewers that ex vivo results may require in vivo confirmation. We have added a sentence explaining the rationale for use of both Balb/c and C57BL/6 mice in the results section “Vitamin D suppresses the activation of the IL-2/Stat5 pathway and cytokine production in Th2 cells”: “To ensure that the above findings were not restricted to the C57BL/6 mouse strain, the inverse experiment was performed in Balb/c mice. This mouse strain is commonly used for type 2 driven inflammation.”
- Certain sections, especially those connecting VDR, Ikzf1/3 and IL2/STAT axis seem associative. This is indicated by Figure 5 H as well, where the effects of calcitriol administration in KO cells indicate additional pathways at play, possibly through indirect effects.
We appreciate this comment. The RNA-Seq results showed an over representation of the IL-2/STAT5 pathway in Vit-D deficient Th2 cells compared to those under Vitamin D supplementation. We further show the induction of IKZF3 expression with calcitriol stimulation. High IKZF3 expression is known to suppress IL-2 expression. Lack of IKZF3 diminishes the suppressive activity of calcitriol on IL-2 expression. However, as pointed out by the reviewer, Figure 5 H implicates additional pathways regulated by calcitriol for the suppression of IL-2 and we note that in the text.
- The use of additional techniques like ChIP, co-IP and establishing STAT induction/activation would probably strengthen the findings, alternatively, a clear distinction between the speculative and the definitive results could be made in the discussion section, as the journal encourages. Similar considerations could be made for VDR and Ikzf3.
We have added the following sentence to the discussion. We have focused here on establishing the relationship between VDR binding and IKFZ3 activation or repression and subsequent ORMDL3 and Il2 activation. Additional use of ChIP or co-IP to establish STAT induction and activation would have been of potential value.
- Role of other cells:
a. While the investigators have explored the phenotype on other cell types like Th1 and Treg, at places there remains a lacuna. For instance, the absence of neutrophil fractions from the DLC BAL, as well inconsistencies in the groups selected for comparison. For e.g., in Figure 3 Supplementary Figure 2, the figure suggests IL13 expression in CD4+ cells, yet the text reads incubated Th2 cells. This could be made more lucid.
We appreciate this comment and would like to clarify. Neutrophil numbers were assessed in the presented in vivo models and showed no differences in neutrophil number due to genotype or vitamin D diet. We added the graphs to the supplement in Figure 3 - figure supplement 1A and Figure 5 - figure supplement 1B and refer to the figures in the main text. All in vivo data were analyzed by Mixed-effect ANOVA analysis or Two-way ANOVA test with Holm-Šidák’s post-hoc analysis (factors: genotype & exposure). To keep the plots clear, we incorporated only the statistic for the groups of interest.
- b) In Figure 3 Supplementary Figure 1 there is a trend towards an increase in IL-10 levels, whereas in Supplementary Figure 2 there is a drop in the IL13 level in the VDR KO group, which has not been explained.
We apologize for any confusion. Figure 3 supplementary Figure 1 shows cytokine positive CD4+ T cells isolated from saline and HDM exposed mouse lungs. These data were analyzed with a Mixed-effect ANOVA analysis or Two-way ANOVA test with Holm-Šidák’s post-hoc analysis (factors: genotype & exposure) and were not found significant. Figure 3 supplementary Figure 2 shows IL-13 levels in the system of in vitro polarization of naïve CD4+ T cells into Th2 cells. The difference between this result and the findings in Figure 3H is the in vivo setting in which additional factors such as IL-4 can aggravate the immune response.
- c) While 17q loci form the predominant loci associated with Asthma, other loci important in Asthma on chromosomes 2,6,9, 22 could be discussed in the manuscript as well, even if they can't be explored in depth.
This is an excellent comment. Our preliminary results confirm that three asthma susceptibility loci: 2q12.1 (IL1RL1), 6p21.32 (HLA-DQA1/B1/A2/B2) and 22q12.3 (IL2RB) each have VDR and IKZF3 binding sites either in enhancers predicted by GeneHancer to target these genes or within these genes themselves. In particular, we found (i) VDR binding sites within IL18RAP and in the enhancer region GH02J102301 targeting IL1RL1, and IKZF3 binding sites within IL1RL1; (ii) VDR binding sites in the enhancer regions GH06J032940 and GH06J031813 targeting HLA-DQA2, and IKZF3 binding sites within HLA-DQA1; (iii) VDR and IKZF3 binding sites within IL2RB. In contrast, the region 9p24.1 (IL33) has no documented VDR or IKZF3 binding sites within IL33 or in the promoter regions targeting IL33. Investigating these additional genetic loci further, using the integrative approach taken here with 17q12-21, is beyond the scope of this current manuscript but based on these preliminary results, would be a worthwhile scientific endeavor.
- Quantification of histology and confocal images could provide an objective assessment to the readers. Possibly incorporation of co-localisation panels for the IF images showing membrane/cytoplasmic/nuclear localisation of the VDR under various conditions.
We agree that quantification of histology and confocal images could provide an overview of VDR expression in the lungs. Given the knowledge on VDR expression in a variety of cell types, including structural cells in the lungs and the focus of this manuscript on CD4+ T cells, we focused on determining VDR expression in CD4+ T cells isolated from saline and HDM exposed lungs in the mouse models studied (Figure 2 C; Fig. 2- figure supplement 1 B & C, Figure 3 C; Figure 5 - figure supplement 1) as well as in vitro (Figure 2 - figure supplement 2; Figure 5 - figure supplement 2).
- Structure of the manuscript: At places the manuscript has a disrupted flow, as well as mislabeled figures (Figure 2SF1B is 1C, Fig 2c is 2b in the results, ). Flow gates can be arranged sequentially and consistent labelling of the gates and axis would ease interpretation.
We appreciate this comment and have corrected the mislabeled figures and tried to improve the flow.
- In some places sample sizes mentioned do not match the dot graphs in the figures (figure 3K-L). In the same figure and others (Figure 5 Supplementary Figure 2), a comparison of all groups would be beneficial.
We appreciate this comment and have checked the sample sizes. Each of these experiments compared two groups and these two groups were compared statistically.
We corrected the sample size for Figure 5 Supplementary Figure 2 C in the manuscript.
- A restructuring of the results and corrections, could assist the reader.
We have restructured both the results and the discussion, incorporating the changes noted here in the response to the reviewers, to make the flow of the manuscript easier to read.
- Also, a visualization of the VDAART analysis in the main figures, corroborating with the results sections would do justice to the interesting approach and findings.
We have now added the below schematic to Figure 1-figure supplement 1C to summarize the analyses conducted on the VDAART data.
Author response image 1.
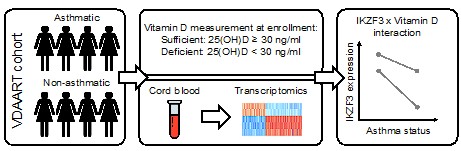
- The clearances and approvals for the study also need to be incorporated into the manuscript.
These were in the checklist and have been moved to the main text of the manuscript.
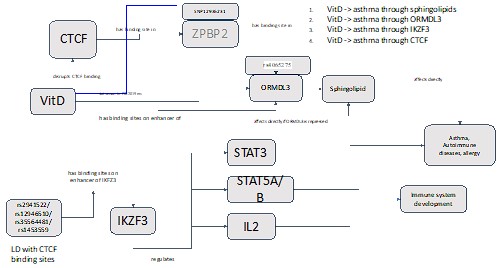
- If possible, the incorporation of a schematic showing the proposed pathway for VDR induced Ikzf3 and subsequent suppression of the genes present on Chr 17 loci to mitigate allergic airway inflammation would help.
We have a figure for this (below) that we have incorporated into the manuscript as Figure 5 - figure supplement 3:
Author response image 2.
Cartoon Summarizing Vitamin D molecular genetics at 17q12-21
Reviewer #2
- A few specific points: A number of immune concepts are studied without reference to the broader literature and the data presented data on occasion counter these earlier findings. Examples of this include:
a. Vitamin D can both enhance and inhibit IL-13 synthesis, demonstrated both in vitro and ex vivo, and these effects are clearly context-specific. I am not questioning the validity of the present experimental findings in this specific experimental model), but the experimental context - the problem is that this is not discussed.
We thank the reviewer for this comment. We have now included a sentence in the discussion section mentioning the contradictory results. It reads as follows:
“We acknowledge that the impact of vitamin D on Th2 biology is conflicting in the literature. While several groups report Th2 promoting activity, we, and others, show inhibition of type 2 cytokine production 8–11. These discrepancies could be due to the model system studied, e.g., PBMC and purified CD4+ T cells, or the dose of vitamin D or the mouse strain.”
b. Short-term bulk Th2 cultures are used with no indication of their enrichment for lineage specific markers or cytokine – their conclusions might be enhanced by this. Data on genes/markers of interest could be further enhanced by showing FACS plots of co-expression e.g., Th2 genes e.g., IL-13/GATA3 with these other markers.
We appreciate this comment. The in vitro culture system used for Th2 cell differentiation has been well described in the literature. As shown in Figure 3 - figure supplement 2; Figure 4 E and Figure 5 - figure supplement 2 D & E the lineage specific IL-13 cytokine levels are detectable at high levels.
c. Are human Th2 cells enriched for VDR, since the backdrop to this study is human clinical and genetic data? For a study that has based its rationale on human clinical/genetic studies it would be great to confirm these findings in human Th2 cells.
We appreciate this comment and are curious to explore this in future research. The VDAART trial is a double-blinded multicenter trial in which an immediate processing of the blood samples and an enrichment of different immune cell populations was not feasible. Other publicly available data sets report gene expression derived from mixed and peripheral (blood) cells and not local (lung) tissues. Published in vitro studies on human Th2 cells do not report VDR expression in comparison to other Th subsets, which would allow the assessment of enrichment.
- The Discussion might comment on some of these wider issues.
We have rewritten the discussion to incorporate many of the issues raised in this review.
- Minor typos throughout, including in figure legends.
We have edited all of the figure legends.
References
Holick, M. F. Vitamin D Is Not as Toxic as Was Once Thought: A Historical and an Up-to-Date Perspective. Mayo Clinic proceedings 90, 561–564; 10.1016/j.mayocp.2015.03.015 (2015).
Hossein-nezhad, A. & Holick, M. F. Vitamin D for health: a global perspective. Mayo Clinic proceedings 88, 720–755; 10.1016/j.mayocp.2013.05.011 (2013).
Hollis, B. W. & Wagner, C. L. New insights into the vitamin D requirements during pregnancy. Bone research 5, 17030; 10.1038/boneres.2017.30 (2017).
Litonjua, A. A. et al. Effect of Prenatal Supplementation With Vitamin D on Asthma or Recurrent Wheezing in Offspring by Age 3 Years: The VDAART Randomized Clinical Trial. JAMA 315, 362–370; 10.1001/jama.2015.18589 (2016).
Gianforcaro, A., Solomon, J. A. & Hamadeh, M. J. Vitamin D(3) at 50x AI attenuates the decline in paw grip endurance, but not disease outcomes, in the G93A mouse model of ALS, and is toxic in females. PloS one 8, e30243; 10.1371/journal.pone.0030243 (2013).
Landel, V., Millet, P., Baranger, K., Loriod, B. & Féron, F. Vitamin D interacts with Esr1 and Igf1 to regulate molecular pathways relevant to Alzheimer's disease. Molecular neurodegeneration 11, 22; 10.1186/s13024-016-0087-2 (2016).
Agrawal, T., Gupta, G. K. & Agrawal, D. K. Vitamin D supplementation reduces airway hyperresponsiveness and allergic airway inflammation in a murine model. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology 43, 672–683; 10.1111/cea.12102 (2013).





