Peer review process
Revised: This Reviewed Preprint has been revised by the authors in response to the previous round of peer review; the eLife assessment and the public reviews have been updated where necessary by the editors and peer reviewers.
Read more about eLife’s peer review process.Editors
- Reviewing EditorAna DomingosUniversity of Oxford, Oxford, United Kingdom
- Senior EditorDavid JamesThe University of Sydney, Sydney, Australia
Reviewer #1 (Public Review):
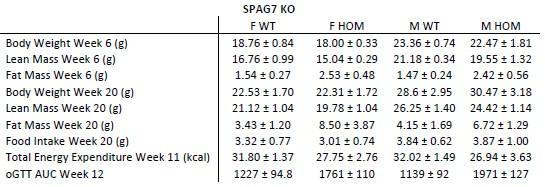
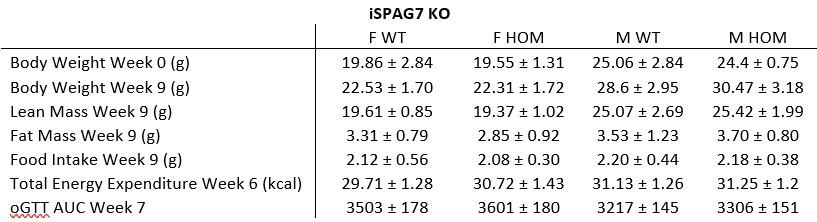
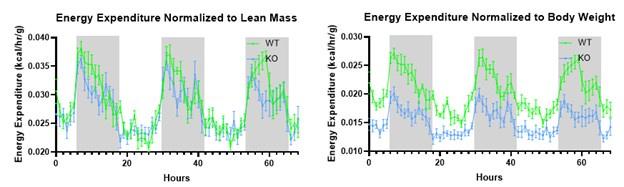
Drawing on insights from preceding studies, the researchers pinpointed mutations within the spag7 gene that correlate with metabolic aberrations in mice. The precise function of spag7 has not been fully described yet, thereby the primary objective of this investigation is to unravel its pivotal role in the development of obesity and metabolic disease in mice. First, they generated a mice model lacking spag7 and observed that KO mice exhibited diminished birth size, which subsequently progressed to manifest obesity and impaired glucose tolerance upon reaching adulthood. This behaviour was primarily attributed to a reduction in energy expenditure. In fact, KO animals demonstrated compromised exercise endurance and muscle functionality, stemming from a deterioration in mitochondrial activity. Intriguingly, none of these effects was observed when using a tamoxifen-induced KO mouse model, implying that Spag7's influence is predominantly confined to the embryonic developmental phase. Explorations within placental tissue unveiled that mice afflicted by Spag7 deficiency experienced placental insufficiency, likely due to aberrant development of the placental junctional zone, a phenomenon that could impede optimal nutrient conveyance to the developing fetus. Overall, the authors assert that Spag7 emerges as a crucial determinant orchestrating accurate embryogenesis and subsequent energy balance in the later stages of life.
The study boasts several noteworthy strengths. Notably, it employs a combination of animal models and a thorough analysis of metabolic and exercise parameters, underscoring a meticulous approach. Furthermore, the investigation encompasses a comprehensive evaluation of fetal loss across distinct pregnancy stages, alongside a transcriptomic analysis of skeletal muscle, thereby imparting substantial value. Upon addressing the previously mentioned aspects, the study is poised to exert a substantial influence on the field, its significance reverberating significantly. The methodologies and data presented undoubtedly hold the potential to facilitate the community's deeper understanding of the ramifications stemming from disruptions during pregnancy, shedding light on their enduring impact on the metabolic well-being of subsequent generations.
Reviewer #2 (Public Review):
Summary:
The authors of this manuscript are interested in discovering and functionally characterizing genes that might cause obesity. To find such genes, they conducted a forward genetic screen in mice, selecting strains which displayed increased body weight and adiposity. They found a strain, with germ-line deficiency in the gene Spag7, which displayed significantly increased body weight, fat mass, and adipose depot sizes manifesting after the onset of adulthood (20 weeks). The mice also display decreased organ sizes, leading to decreased lean body mass. The increased adiposity was traced to decreased energy expenditure at both room temperature and thermoneutrality, correlating with decreased locomotor activity and muscle atrophy. Major metabolic abnormalities such as impaired glucose tolerance and insulin sensitivity also accompanied the phenotype. Unexpectedly, when the authors generated an inducible, whole body knockout mouse using a globally expressed Cre-ERT2 along with a globally floxed Spag7, and induced Spag7 knockout before the onset of obesity, none of the phenotypes seen in the original strain were recapitulated. The authors trace this discrepancy to the major effect of Spag7 being on placental development.
Strengths:
Strengths of the manuscript are its inherently unbiased approach, using a forward genetic screen to discover previously unknown genes linked to obesity phenotypes. Another strong aspect of the work was the generation of an independent, complementary, strain consisting of an inducible knockout model, in which the deficiency of the gene could be assessed in a more granular form. This approach enabled the discovery of Spag7 as a gene involved in the establishment of the mature placenta, which determines the metabolic fate of the offspring. Additional strengths include the extensive array of physiological parameters measured, which provided a deep understanding of the whole-body metabolic phenotype and pinpointed its likely origin to muscle energetic dysfunction.
Weaknesses:
Weaknesses that can be raised are the lack of molecular mechanistic understanding of the numerous phenotypic observations. For example, the specific role of Spag7 to promote placental development remains unclear. Also, the reason why placental developmental abnormalities lead to muscle dysfunction, and whether indeed the entire metabolic phenotype of the offspring can be attributed solely to decreased muscle energetics is not fully explored.
Overall, the authors achieved a remarkable success in identifying genes associated with development of obesity and metabolic disease, discovering the role of Spag7 in placental development, and highlighting the fundamental role of in-utero development in setting future metabolic state of the offspring.
Comments on revised version:
I have no further comments on my assessment of this interesting paper.
Reviewer #3 (Public Review):
Summary:
The manuscript by Flaherty III S.E. et al identified SPAG7 gene in their forward mutagenetic screening and created the germline knockout and inducible knockout mice. The authors reported that the SPAG7 germline knockout mice had lower birth weight likely due to intrauterine growth restriction and placental insufficiency. The SPAG7 KO mice later developed obesity phenotype as result of reduced energy expenditure. However, the inducible SPAG7 knockout mice had normal body weight and composition.
Strengths:
In this reviewer's opinion, this study has high significance in the field of metabolic research for the following reasons.
The authors' findings are significant in the field of obesity research, especially from the perspective of maternal-fetal medicine. The authors created and analyzed the SPAG7 KO mice and found that the KO mice had a "thrifty phenotype" and developed obesity.
SPAG7 gene function hasn't been thoroughly studied. The reported phenotype will fill the gap of knowledge.
Overall, the authors have presented their results in a clear and logically organized structure, clearly stated the key question to be addressed, used the appropriate methodology, produced significant and innovative main findings.
Comments on revised version:
The authors have satisfactorily addressed my previous concerns.