Peer review process
Revised: This Reviewed Preprint has been revised by the authors in response to the previous round of peer review; the eLife assessment and the public reviews have been updated where necessary by the editors and peer reviewers.
Read more about eLife’s peer review process.Editors
- Reviewing EditorDetlef WeigelMax Planck Institute for Biology Tübingen, Tübingen, Germany
- Senior EditorDetlef WeigelMax Planck Institute for Biology Tübingen, Tübingen, Germany
Reviewer #1 (Public Review):
Summary:
Overall, this study provides a meticulous comparison of developmental transcriptomes between two sub-species of the annelid Streblospio benedicti. Different lineages of S. benedicti maintain one of two genetically programmed alternative life histories, the ancestral planktotrophic or derived lecithotrophic forms of development. This contrast is also seen at the inter-species level in many marine invertebrate taxa, such as echinoderms and molluscs. The authors report relatively (surprisingly?) modest differences in transcriptomes overall, but also find some genes whose expression is essentially morph-specific (which they term "exclusive").
Strengths:
The study is based on dense and appropriately replicated sampling of early development. The tight clustering of each stage/morph combination in PCA space suggests the specimens were accurately categorized. The similar overall trajectories of the two morphs was surprising to me for two stage: 1) the earliest stage (16-cell), at which we might expect maternal differences due to the several-fold difference in zygote size, and 2) the latest stage (1-week), where there appears to be the most obvious morphological difference. This is why we need to do experiments!
The examination of F1 hybrids was another major strength of the study. It also produced one of the most surprising results: though intermediate in phenotype, F1 embryos have the most distinct transcriptomes, and reveal a range of fixed, compensatory differences in the parental lines. Further, the F1 lack expression of nearly all transcripts identified as morph-specific in the pure parental lines. Since the F1 larvae present intermediate traits combining the features of both morphs, this implies that morph-specific transcripts are not actually necessary for morph-specific traits. This is interesting and somewhat counter to what one might naively expect.
Weaknesses:
Overall I really enjoyed this paper, and in its revised form it addresses some concerns I had in the first version. I still see a few places where it can be tightened and made more insightful.
Reviewer #2 (Public Review):
The manuscript by Harry and Zakas determined the extent to which gene expression differences contribute to developmental divergence by using a model that has two distinct developmental morphs within a single species. Although the authors did collect a valuable dataset and trends in differential expression between the two morphs of S. benedicti were presented, we found limitations about the methods, system, and resources that the authors should address.
We have two major points:
(1) Background information about the biological system needs to be clarified in the introduction of this manuscript. The authors stated that F1 offspring can have intermediate larval traits compared to the parents (Line 81). However, the authors collected F1 offspring at the same time as the mother in the cross. If offspring have intermediate larval traits, their developmental timeline might be different than both parents and necessitate the collection of offspring at different times to obtain the same stages as the parents. Could the authors (1) explain why they collected offspring at the same time as parents given that other literature and Line 81 state these F1 offspring develop at intermediate rates, and (2) add the F1 offspring to Figure 1 to show morphological and timeline differences in development?
Additionally, the authors state (Lines 83-85) that they detail the full-time course of embryogenesis for both the parents and the F1 crosses. However, we do not see where the authors have reported the full-time course for embryogenesis of the F1 offspring. Providing this information would shape the remaining results of the manuscript.
(2) We have several concerns about the S. benedicti genome and steps regarding the read mapping for RNA-seq:
The S. benedicti genome used (Zakas et al. 2022) was generated using the PP morph. The largest scaffolds of this assembly correspond to linkage groups, showing the quality of this genome. The authors should point out in the Methods and/or Results sections that the quality of this genome means that PP-specific gene expression can be quantified well. However, the challenges and limitations of mapping LL-specific expression data to the PP genome should be discussed.
It is possible that the authors did not find exclusive gene expression in the LL morph because they require at least one gene to be turned on in one morph as part of the data-cleaning criteria. Because the authors are comparing all genes to the PP morph, they could be missing true exclusive genes responsible for the biological differences between the two morphs. Did they make the decision to only count genes expressed in one stage of the other morph because the gene models and mapping quality led to too much noise?
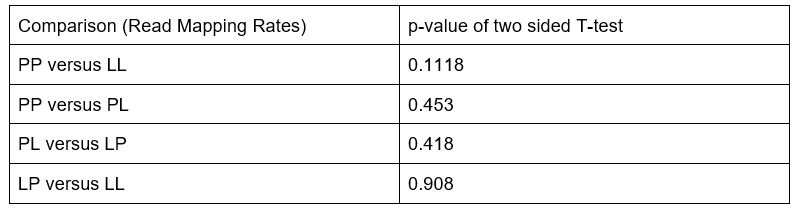
The authors state that the mapping rates between the two morphs are comparable (Supplementary Figure 1). However, there is a lot of variation in mapping the LL individuals (~20% to 43%) compared to the PP individuals. What is the level of differentiation within the two morphs in the species (pi and theta)? The statistical tests for this comparison should be added and the associated p-value should be reported. The statistical test used to compare mapping rates between the two morphs may be inappropriate. The authors used Salmon for their RNA alignment and differential expression analysis, but it is possible that a different method would be more appropriate. For example, Salmon has some limitations as compared to Kallisto as others have noted. The chosen statistical test should be explained, as well as how RNA-seq data are processed and interpreted.
What about the read mapping rate and details for the F1 LP and PL individuals? How did the offspring map to the P genome? These details should be included in Supplementary Figure 1. Could the authors also provide information about the number of genes expressed at each stage in the F1 LP and PL samples in S Figure 2? How many genes went into the PCA? Many of these details are necessary to evaluate the F1 RNA-seq analyses.
Generally, the authors need to report the statistics used in data processing more thoroughly. The authors need to report the statistics used to (1) process and evaluate the RNA-seq data and (2) determine the significance between the two morphs (Supplementary Figures 1 and 2).