Cell-cycle dependent phosphorylation of yeast pericentrin regulates γ-TuSC-mediated microtubule nucleation
Peer review process
This article was accepted for publication as part of eLife's original publishing model.
History
- Version of Record published
- Accepted Manuscript published
- Accepted
- Received
Decision letter
-
Jon PinesReviewing Editor; The Gurdon Institute, United Kingdom
eLife posts the editorial decision letter and author response on a selection of the published articles (subject to the approval of the authors). An edited version of the letter sent to the authors after peer review is shown, indicating the substantive concerns or comments; minor concerns are not usually shown. Reviewers have the opportunity to discuss the decision before the letter is sent (see review process). Similarly, the author response typically shows only responses to the major concerns raised by the reviewers.
Thank you for sending your work entitled “Cell-cycle dependent phosphorylation of yeast pericentrin regulates γ-TuSC-mediated microtubule nucleation” for consideration at eLife. Your article has been favorably evaluated by a Senior editor, a Reviewing editor, and 2 reviewers.
The Reviewing editor and the reviewers discussed their comments before we reached this decision, and the Reviewing editor has assembled the following comments to help you prepare a revised submission.
1) The primary concern is with T18. It is likely that this site is phosphorylated in vivo, but Spc110 mutant proteins containing T18D or T18A have the same rather than opposing properties. Therefore, the evidence that that phosphorylation of T18 is really inhibitory is lacking. (It is likely that T18D did not act as a phosphomimetic.) The strong effect of T18Ala is a concern. It is difficult to see why this should perturb the structure, as the authors claim. The authors could try a valine substitution instead.
2) It is puzzling that mitotic recruitment of Spc97 to the SPB of T18D mutants remains unchanged (Figure 7–figure supplement 1D), although there is an effect on microtubule nucleation. This also contrasts with the effect of T18 phosphorylation on the oligomerization of gamma-TuSCs seen in vitro. It is important to resolve this discrepancy.
3) It would further strengthen this manuscript if the authors could demonstrate in a more refined way that the pT18 epitope peaks late in mitosis and is lost upon mitotic exit (e.g., by immunoblotting of synchronized cells with anti pT18 in a time course experiment). The anti-phospho-T18 blots in Figure 6A are not convincing.
4) The biological significance of T18 phosphorylation to inhibit microtubule nucleation is unclear. The dynamics of spindle microtubules are high during this mid-mitosis, thus to support this property it is likely that nucleation activities from the SPB should be high as well.
https://doi.org/10.7554/eLife.02208.028Author response
1) The primary concern is with T18. It is likely that this site is phosphorylated in vivo, but Spc110 mutant proteins containing T18D or T18A have the same rather than opposing properties. Therefore, the evidence that that phosphorylation of T18 is really inhibitory is lacking. (It is likely that T18D did not act as a phosphomimetic.) The strong effect of T18Ala is a concern. It is difficult to see why this should perturb the structure, as the authors claim. The authors could try a valine substitution instead.
As suggested by the reviewers, we have constructed spc110-T18V and in addition, spc110-T18E mutants. We have analyzed both mutants using biochemistry (Figure 2–figure supplement 5A) and cell growth (Figure 2–figure supplement 5B). The bottom line of both experiments is that spc110-T18A behaves as spc110-T18V and spc110-T18D behaves as spc110-T18E.
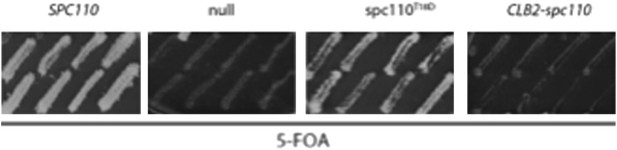
In addition, we have constructed a destruction box lacking ΔDB-CLB2-SPC110 gene fusion following a strategy that was originally introduced by D. Morgan. We hoped that this gene fusion will target Cdk1 permanently to the SPB and phosphorylate T18 in a cell cycle independent manner. This should give us the spc110-pT18 phenotype. However, the CLB2-SPC110 gene fusion did not provide SPC110 function (Author response image 1.)
To this end, we cannot say without ambiguities that T18D/E are phosphomimetic and T18A/V are phosphoinhibitory. However, it is clear that T18 lies within a conserved region, that we named SPM. Thus, we have changed the T18 phosphorylation part in this direction. In Figure 5 – figure supplement 1B we now show that T18 of Spc110 is phosphorylated in mitosis and we also show the in vitro data on Spc1101-220-5D-pT18 on γ-TuSC oligomerization (Figure 5– figure supplement 1D, E). These data are consistent with an inhibitory role of pT18. This role is now discussed in the Discussion.
2) It is puzzling that mitotic recruitment of Spc97 to the SPB of T18D mutants remains unchanged (Figure. 7–figure supplement 1D), although there is an effect on microtubule nucleation. This also contrasts with the effect of T18 phosphorylation on the oligomerization of gamma-TuSCs seen in vitro. It is important to resolve this discrepancy.
In response to this comment, we have performed the experiment shown in Figure 6–figure supplement 1D. It shows that Spc97-GFP is recruited to SPBs of spc110-T18D cells with a time delay after SPB duplication. However, despite equal Spc97 amounts at SPBs in mitosis, spc110-T18D cells have a MT organization defect (Figure 6B). Indeed, Figure 2–figure supplement 2A shows that Spc1101-220-T18D and Spc1101-220-5D-T18D form putative dimers with γ-TuSC (shoulder at 600 kDa) without inducing γ-TuSC oligomerization (Vo). This indicates that γ-TuSC recruitment to SPBs via Spc110 and γ-TuSC oligomerization into a nucleation platform are mechanistically two distinct steps. We discuss this scenario in the Discussion.
3) It would further strengthen this manuscript if the authors could demonstrate in a more refined way that the pT18 epitope peaks late in mitosis and is lost upon mitotic exit (e.g., by immunoblotting of synchronized cells with anti pT18 in a time course experiment). The anti-phospho-T18 blots in Figure 6A are not convincing.
We tried to detect pT18 of Spc110 in IB with the P-specific anti-pT18 antibodies. However, this antibody only works with in vitro phosphorylated Spc110-pT18 (Figure 5–figure supplement 1A). We therefore turned to mass spectrometry to determine the cell cycle dependent phosphorylation of Spc110. We have fractionated yeast cells into a supernatant fraction and a SPB containing pellet. We have extracted SPB proteins from the pellet. Spc110-GFP was enriched form the supernatant and the SPB faction. As shown in Figure 5–figure supplement 1B,C T18 of Spc110 is predominately phosphorylated in mitosis. This fits with the data from Keck et al., which showed that Spc110-T18 was phosphorylated in mitosis.
4) The biological significance of T18 phosphorylation to inhibit microtubule nucleation is unclear. The dynamics of spindle microtubules are high during this mid-mitosis, thus to support this property it is likely that nucleation activities from the SPB should be high as well.
As outlined under 1) we have changed this part of the paper. In budding yeast, nuclear MTs are only dynamic from the plus end because of the blocking cap of γ-TuSC at the MT minus end. Data from T. Stearns (Murphy et al., 1998) suggest that in budding yeast MT nucleation mainly happens in S phase when SPBs duplicate but probably not in other cell cycle phases. The T18 phosphorylation could inhibit excess MT nucleation by SPB-associated γ-TuSC in mitosis. SPBs organize relative constant numbers of 20 MTs in mitosis (Winey et al., 1995).
Because T18A is not behaving as a non-phosphorylatable mutation, we unfortunately cannot test this model. However, we have discussed in line 510 of the Discussion the possibility that pT18 restricts the number of nuclear MTs in mitosis.
https://doi.org/10.7554/eLife.02208.029