The C-terminal region of the motor protein MCAK controls its structure and activity through a conformational switch
Figures
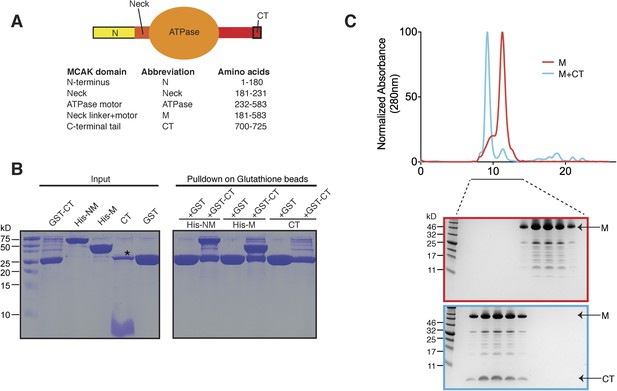
The C terminus of MCAK binds to the motor domain.
(A) Top: schematic diagram showing the different functional domains of full-length MCAK. Bottom: table representing the constructs used and given names. (B) Coomassie-stained gel showing a resin-based binding assay for purified His-M, His-NM, and CT domains to either glutathione agarose beads containing GST (as a control) or the GST-CT domain. The star represents residual GST. (C) Top, gel filtration elution profile of MCAK motor alone (M, red) and MCAK motor bound to the CT domain (M + CT, cyan). Bottom, coomassie-stained gel showing the size-exclusion chromatography profile of M and M + CT.
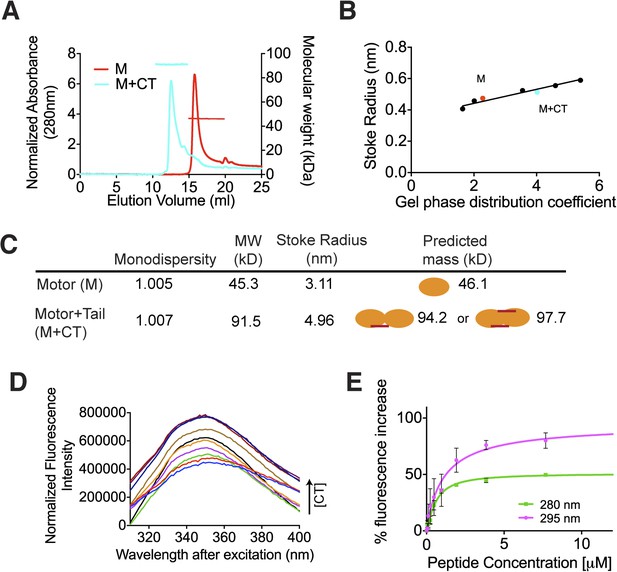
The C terminus of MCAK induces motor domain dimerization.
(A) Size-exclusion chromatography elution profiles of motor domain alone (red) and motor domain-CT domain complex (cyan). The horizontal red and cyan lines correspond to SEC-MALS calculated masses for motor domain alone and motor domain-CT domain complex, respectively. (B) Calibration curve for estimation of Stokes radii of motor domain alone (red) and motor domain-CT domain complex (cyan). (C) Table to show the calculated apparent masses and stoke radii of the motor domain-CT domain complex and motor domain alone. The motor domain is drawn in orange and the CT domain in red, to represent the formation of the possible complexes and their predicted size. (D) Steady state intrinsic tryptophan fluorescence emission spectra profile for the titration of the CT domain (CT) ranging from 0 to 15.6 μM, into 1 μM of motor domain after excitation at 295 nm. (E) Effect of the CT domain titration on tryptophan (magenta) and aromatic residue (green) fluorescence quenching of the motor domain. The extent of fluorescence quenching of the motor domain is represented as a percentage of fluorescence change measured for aromatic residues (280 nm) and tryptophan (295 nm) with increasing concentration of wild type CT domain. Relative change in fluorescence after background correction is shown as a function of CT domain concentration. Error bars represent the standard deviation.
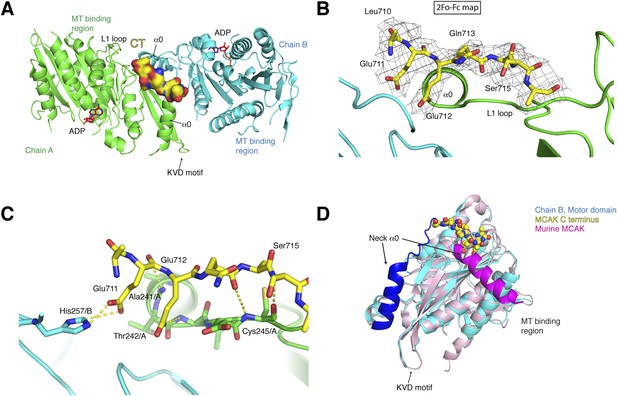
Structure of a human motor-CT domain MCAK complex.
(A) Kinesin motor domain dimers (cyan and green) bound to the CT domain (yellow, spacefill) of MCAK. ADP is in red. (B) Motor-CT domain interface showing the electron density map (2Fobs − Fcalc), contoured at σ = 1.00 for the CT domain of MCAK. (C) Interactions within the motor-CT domain complex of less than 4 Å are represented by dotted lines. Oxygen and nitrogen atoms are colored red and blue. (D) Overlay of the human motor domain and C terminus structure (blue) with the structure of murine MCAK (pink, PDB: 1V8J). The respective neck regions containing the α0 and neck linker are in royal blue and magenta, respectively. The CT domain of MCAK is drawn in yellow as a sphere model with oxygen and nitrogen atoms in blue and red.
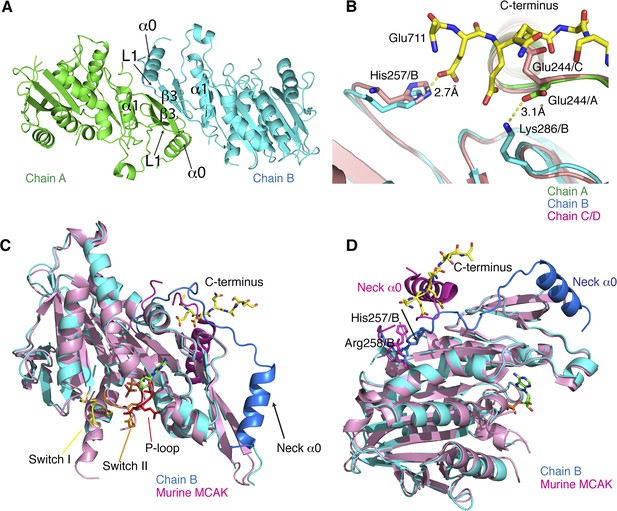
Structural analysis of the MCAK motor-CT domain.
(A) Dimeric interface of MCAK motors (cyan and green). The respective neck regions containing α0 and loop L1 and the dimerization interface including α1 and β3 are indicated. (B) Overlay of chain A (green) and B (blue) with chain C and D (salmon), showing that Glu244 in chain C points towards the peptide binding site. Glu244/A is repositioned and stabilized by Lys286/B through a salt bridge interaction in presence of the CT domain. His257/B is also repositioned in chain B in the presence of the CT domain. (C) Overlay of our MCAK motor-CT domain structure (cyan) with the structure of murine MCAK (pink, PDB: 1V8J) showing the switch I (yellow), switch II regions (orange), and the ATP-binding P-loop site (red). The neck regions are shaded in darker blue and pink, respectively. (D) Orientation of the neck regions for overlaid mouse and human MCAK structures (pink and blue, respectively). The change in direction of the neck linker occurs around His257 and Arg258.
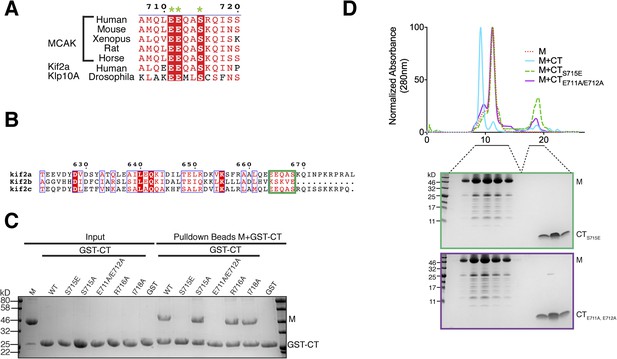
Sequence requirement for the formation of a motor-CT tail complex.
(A) Sequence alignment of the conserved CT domain of MCAK for various species alongside the Drosophila kinesin-13 Klp10A and human Kif2a. The conserved residues are highlighted in red. The three amino acids that are critical for binding to the motor domain are marked with a green star. (B) Sequence alignment of the C terminus of human Kif2a, Kif2b, and MCAK/Kif2c. Amino acid numbering is relative to the Kif2a sequence. The MCAK CT domain binding to the motor domain is boxed in green. The sequences were aligned using the program T-coffee (EBI) and formatted with ESPRIPT (Gouet et al., 1999). (C) Coomassie-stained gel showing a resin-based binding assay using glutathione agarose beads for purified His-M, binding to the GST-CT and GST-CT point mutants. (D) Size-exclusion chromatography elution profile of the motor domain alone (red dashes), motor incubated with the CT, CTS715E, CTE711A, E712A domains (cyan, green dashes, and purple, respectively). Bottom, coomassie-stained gel showing the size-exclusion chromatography elution of the motor incubated with the CTS715E and CTE711A, E712A domains (green and purple, respectively).
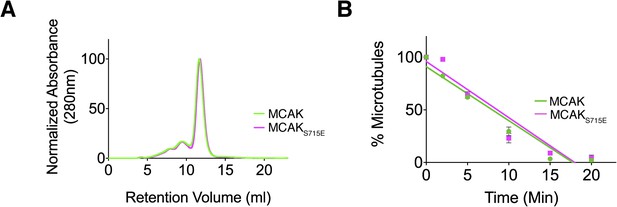
Full-length MCAK remains dimeric upon disruption of the motor-CT tail interaction but retains its depolymerase activity.
(A) Size-exclusion chromatography elution profiles of full-length MCAK (green) and full-length MCAKS715E (magenta). (B) Graph plotting the microtubule depolymerase activity of 100 nM MCAK and MCAKS715E by measuring the distribution of 2 μM microtubules in the pellet (P) and free soluble tubulin (S) over time. Error bars represent the standard deviation. Experiments were repeated three times.
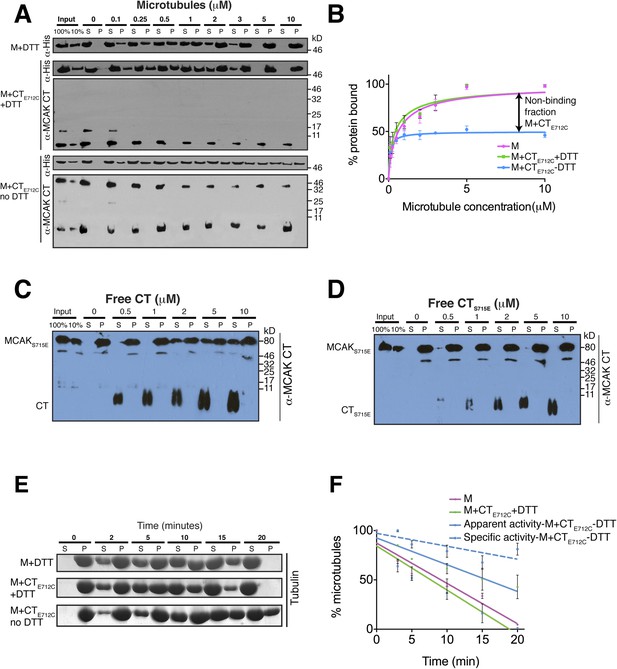
The binding of the CT domain to the motor prevents MCAK binding to microtubules and reduces MCAK depolymerase activity.
(A) Western blot showing the cosedimentation of 50 nM the motor domain of MCAK alone and in the presence of the cleaved CTE712C domain, with and without the addition of DTT to control the formation of the disulphide bridge, at the indicated concentration of microtubules. Detection of the MCAK motor and the CT domain were done using an anti-His and anti-MCAK CT domain antibody, respectively. When the CTE712C domain is covalently bound to the motor domain, we observe free CTE712C domain (∼3.5 kD) and motor-bound CTE712C domain (∼47 kD) when probing for the CT domain. The CTE712C-bound motor remained in the supernatant. (B) Graph plotting the microtubule binding activity of the complexes in (A) in absence of nucleotide. Data were fitted with a modified Hill equation (Welburn et al., 2010). Error bars represent the standard deviation. (C and D) Western blot showing the cosedimentation of 100 nM full-length MCAKS715E incubated in the presence of 2 μM taxol-stabilized microtubules with increasing concentration of the cleaved free CT (C) and CTS715E (D) domains. The western blots were probed with the antibody directed against the CT domain. All experiments were repeated three times. (E) Coomassie-stained gel showing the microtubule depolymerization activity of 50 nM MCAK motor alone and 50 nM MCAK motor-CTE712C domain in presence and absence of DTT, over time on 2 μM taxol-stabilized microtubules. Free tubulin and microtubule polymers were separated using a cosedimentation assay. (F) Graph plotting the quantified microtubule depolymerase activity for conditions in (E). The data were fitted with linear regression. The specific depolymerase activity of a covalent MCAK-CTE712C complex was calculated by subtracting the activity of MCAK motor alone, which represents ∼50% of the population.
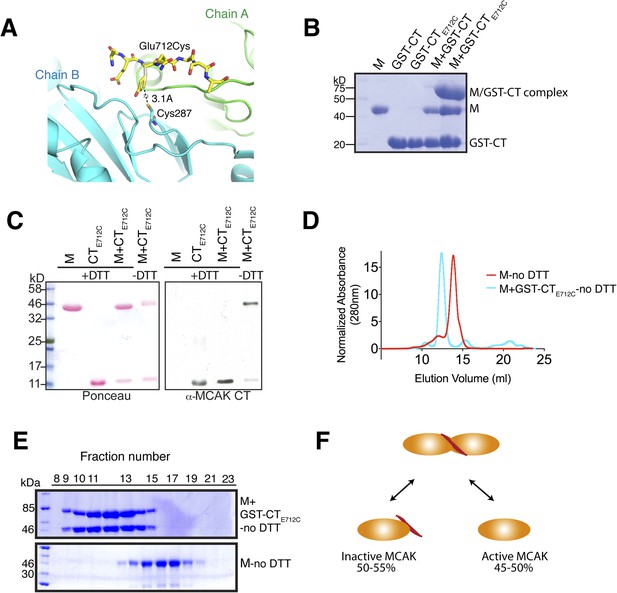
Tunable covalent linkage of the CT domain of MCAK to the motor.
(A) Model showing that mutation of Glutamate 712 to Cysteine can create a disulphide bond between Cysteine 287 and Cysteine 712. (B) Coomassie-stained gel showing that in the absence of reducing agent such as DTT, the GST-CTE712C domain binds specifically the motor domain through a disulphide bridge with a ∼50% efficiency. (C) Western blot probing for the CT domain and ponceau stain showing total protein indicate that in absence of DTT the motor and the CT domain form a covalent complex. There are two bands for the motor domain, one of them coupled to the CT domain, with similar stoichiometry to (B). (D) Coomassie-stained gel showing the size-exclusion chromatography profile of the motor and the GST-CTE712C domain (M + GST-CTE712C). For one motor-CT complex, there is one free motor and one CT-bound motor. (E) Gel filtration elution profile of MCAK motor alone (M, red) and MCAK motor bound to the GST-CTE712C domain (M + GST-CTE712C, cyan). (F) Schematic diagram of the efficiency of disulphide bridge formation for MCAK motor dimers, quantified from (B).
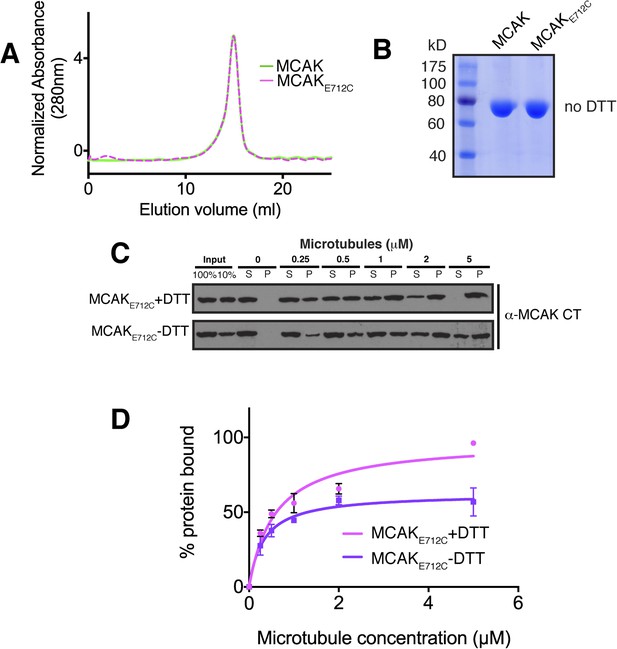
The affinity of MCAK for microtubules decreases when the CT domain of MCAK is not displaced from the motor.
(A) Size-exclusion chromatography elution profile of full-length MCAK (green) and MCAKE712C (magenta) in absence of DTT. (B) Coomassie-stained gel showing MCAK and MCAKE712C in the absence of reducing agent such as DTT. (C) Western blot showing the cosedimentation of 50 nM MCAKE712C in absence of nucleotide, with and without the addition of DTT to control the formation of the disulphide bridge, at the indicated concentration of microtubules. (D) Graph plotting the microtubule binding activity of the proteins in (C) and fitted with a modified Hill equation (Welburn et al., 2010). All experiments were repeated at least three times. Error bars represent the standard deviation.
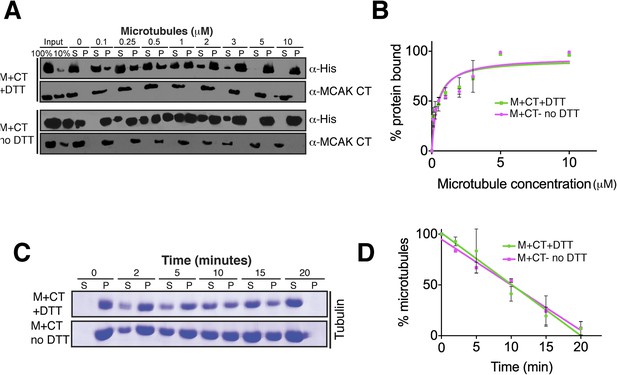
Absence of reducing agent does not affect MCAK motor properties.
(A) Western blot showing the cosedimentation of 50 nM motor domain of MCAK in presence of the CT domain (M + CT) and in absence of nucleotide, with and without the addition of DTT to control the formation of the disulphide bridge, at the indicated concentration of microtubules. (B) Graph plotting the microtubule binding activity of the complexes in (A). (C) Coomassie-stained gel showing the microtubule depolymerization activity of 50 nM MCAK motor with the CT domain in presence and absence of DTT, over time on 2 μM taxol-stabilized microtubules. Free tubulin and microtubule polymers were separated using a cosedimentation assay. (D) Graph plotting the quantified microtubule depolymerase activity for conditions in (C). All experiments were repeated at least three times. Error bars represent the standard deviation.
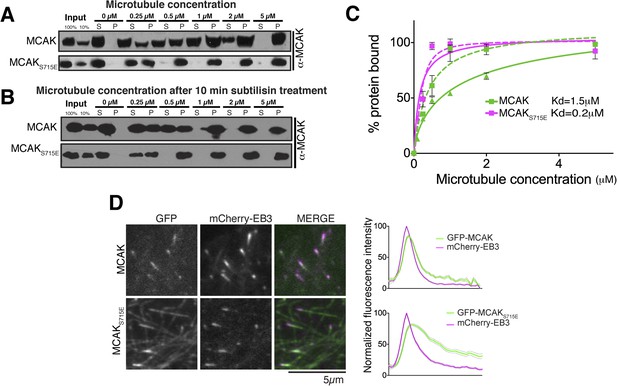
The CT domain reduces the affinity of MCAK to microtubules.
(A and B) Western blot showing the cosedimentation of 50 nM MCAK with microtubules at the indicated concentrations. In panel B, the microtubules have been treated with subtilisin for 10 min prior to the cosedimentation assay. (C) Graph plotting the average microtubule binding activity of MCAK and MCAKS715E in absence of nucleotide. The dashed and full curves correspond to subtilisin-treated and untreated microtubules, respectively. The data were fitted using a modified Hill equation. Error bars represent the standard deviation. (D) Representative images of HeLa cells transiently transfected with mCherry-EB3 and GFP-MCAK or GFP-MCAKS715E, alongside the respective average normalized fluorescence intensity linescan profiles at microtubule plus tips. Grey shading of the linescans represents the standard error.
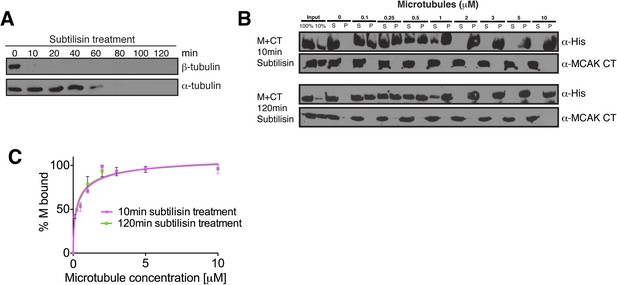
The displacement of the CT domain from the motor is triggered by the microtubule lattice but is independent of the E-hook of tubulin.
(A) Western blot showing the efficiency of the α- and β-tubulin tails removal over time. (B) Western blot showing the cosedimentation of 50 nM MCAK motor domain with microtubules at the indicated concentrations probed with antibodies detecting the C-terminal tails of α- and β-tubulin. The microtubules have been treated with subtilisin for 10 or 120 min prior to the cosedimentation assay. (C) Graph plotting for the average microtubule binding activity of MCAK motor in absence of nucleotide after 10 or 120 min subtilisin-treatment.
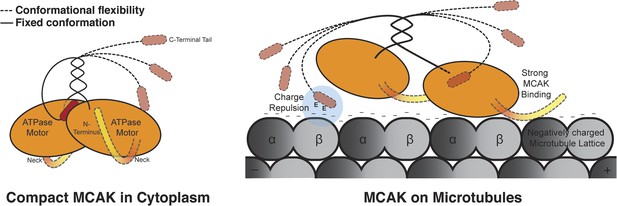
Model for MCAK conformation in solution and when bound to microtubules.
MCAK has a compact structure in solution, with one C terminus binding at the interface between two motor domains. MCAK can bind to microtubules through the microtubule-binding region, which allosterically triggers release of the C-terminus of MCAK. The motor domains can then efficiently bind to and depolymerize the microtubule end, through possible repositioning of the neck linker region. Both the C terminus of MCAK and the negatively charged E-hook of tubulin, reduce the binding of MCAK to microtubules, enabling MCAK to diffuse efficiently to microtubule ends.
Tables
Data collection, structure determination, and refinement statistics for the X-ray crystal structure of the CT domain of MCAK bound to its motor domain
| Statistics | MCAK motor domain-peptide complex |
|---|---|
| Unit cell dimensions | a = 46.31 Å, b = 245.64 Å, c = 79.40 Å, α = 90.00°, β = 95.84°, γ = 90.00° |
| Space group | P21 |
| Molecules per asymmetric unit | 4 |
| Resolution range (Å) | 30.0–3.0 |
| Total reflections | 155983 |
| Unique reflections | 35,146 |
| Completeness (%) | 99.0 (99.2) |
| Multiplicity | 4.4 (4.5) |
| Rsym (%) | 9.1 (68.2) |
| I/σ(I) | 10.2 (2.0) |
| Rwork/Rfree (%) | 26.4/28.6 |
| Wilson B (Å2) | 77.5 |
| Average B (Å2): | |
| Overall | 71.0 |
| Main chain | 72.05 |
| Side chain and solvent | 70.66 |
| Peptide | 56.98 |
| r.m.s.d. bond lengths (Å) | 0.095 |
| r.m.s.d. bond angles (°) | 1.53 |
| Ramachandran plot statistics (%): | |
| Favoured | 87.6 |
| Allowed | 11.7 |
| Outliers | 0.7 |





