Functional evidence implicating chromosome 7q22 haploinsufficiency in myelodysplastic syndrome pathogenesis
Figures
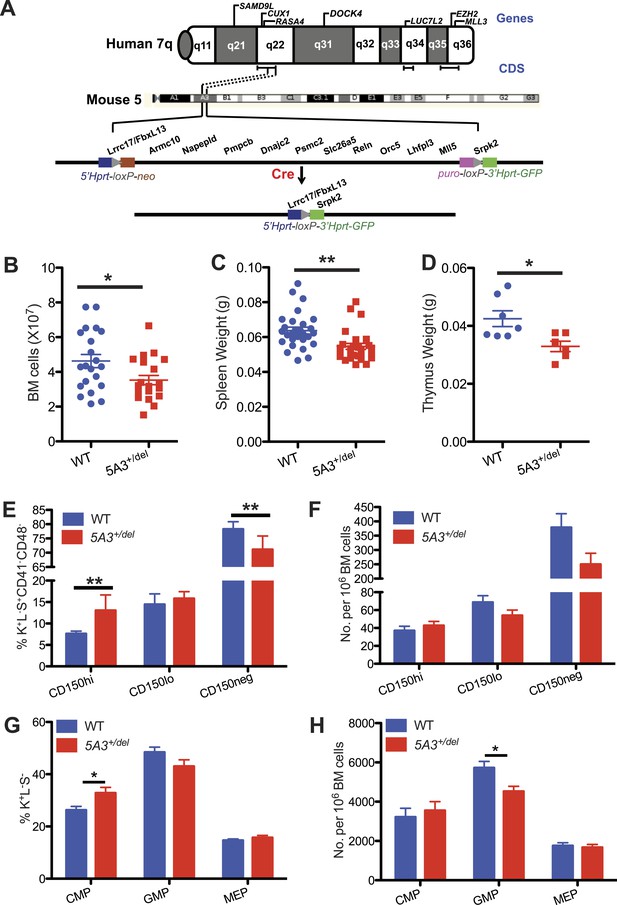
A heterozygous 5A3 deletion corresponding to human 7q22 perturbs steady-state hematopoiesis.
(A) Top, candidate 7q myeloid tumor suppressor genes described previously (Asou et al., 2009; Ernst et al., 2010; Nikoloski et al., 2010; Zhou et al., 2011; McNerney et al., 2013; Chen et al., 2014; Hosono et al., 2014; Poetsch et al., 2014) appear above the diagram of chromosome 7q while commonly deleted segments (CDSs) within 7q22, 7q34, and 7q35-36 identified by different research groups (Kere et al., 1987a; Le Beau et al., 1996; Fischer et al., 1997; Liang et al., 1998; Tosi et al., 1999; Jerez et al., 2012; McNerney et al., 2013; Hosono et al., 2014) are depicted by brackets immediately below it. Middle, dotted lines demarcate the segments of mouse chromosome 5A3 corresponding to the human 7q22 CDS targeted in this study. Bottom, expressing Cre recombinase in embryonic stem (ES) cells doubly targeted with LoxP sequences within the Fbxl13 and Srpk2 genes excised a 2-Mb interval. Gene order is based on the Ensembl database and is not drawn to scale. (B) Total numbers of bone marrow (BM) cells from 2 femurs and 2 tibiae in 5A3+/del mice and wild-type (WT) littermates at 8–12 weeks of age. (C, D) Spleen (C) and thymus (D) weights in 5A3+/del mice and WT littermates at 8–12 weeks of age. (E, F) Percent contributions (E) and frequencies (F) of cells with high (CD150hi HSC), low (CD150lo HSC), and absent CD150 expression (CD150neg MPP) within the K+L−S+CD41−CD48− compartment of WT and 5A3+/del mice at 8–12 weeks of age (n = 6 of each genotype). (G) Percent contribution of common myeloid progenitor (CMP), granulocyte-monocyte progenitor (GMP), and megakaryocyte erythroid progenitors (MEP) within the Lin−Sca1+c-kit+ compartment of 5A3+/del mice and WT littermates. (H) Frequencies of CMP, GMP, and MEP in WT and 5A3+/del BM. The error bars indicate S.E.M. with significant differences between WT and 5A3+/del mice designated by asterisks as follows: *p < 0.05, **p < 0.01.
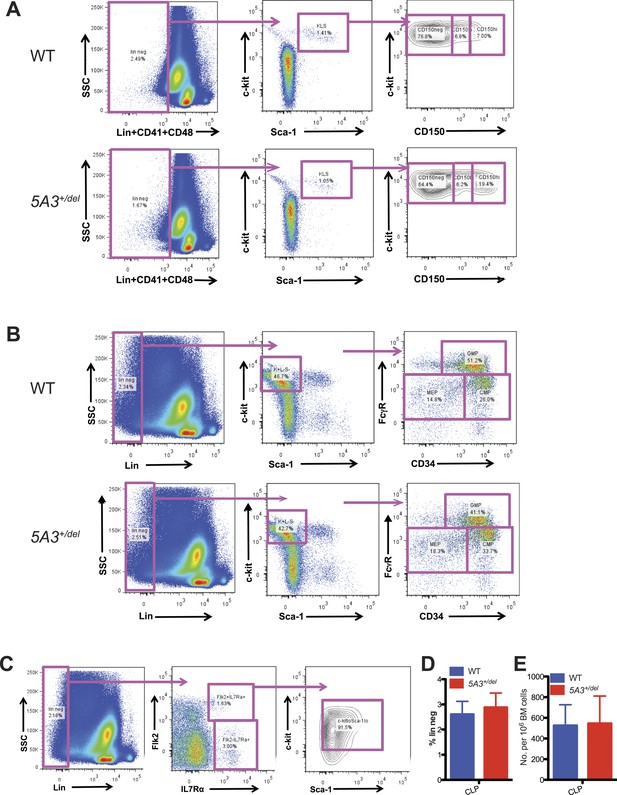
Gating strategy for hematopoietic stem and progenitor populations.
(A) Gating strategy for CD150hi-HSC, CD150lo-HSC, and CD150neg-MPP. K+L−S+CD41−CD48− cells were separated based on CD150 expression into CD150hi, CD150lo, and CD150neg populations. Representative plots from WT (top) and 5A3+/del (bottom) mice are shown. (B) Gating strategy for CMP, GMP, and MEP. K+L−S− cells were subdivided by CD34 and FcγR expression into CMP (CD34+FcγRlo), GMP (CD34+FcγRhi), and MEP (CD34−FcγRlo). Representative plots from WT (top) and 5A3+/del (bottom) mice are shown. (C) Gating strategy for common lymphoid progenitor (CLP). Lin− cells were gated for CLP (Flk2+IL7Rα+). (D) Percent contribution of CLP within the Lin− compartment of WT (n = 6) and 5A3+/del (n = 6) mice. (E) Frequencies of CLP in WT (n = 6) and 5A3+/del (n = 6) BM. For CLP staining experiments, data shown are mean values ±SEM of results from three independent experiments. For gating strategy figures, the numbers are expressed as percentages of the parental gates.
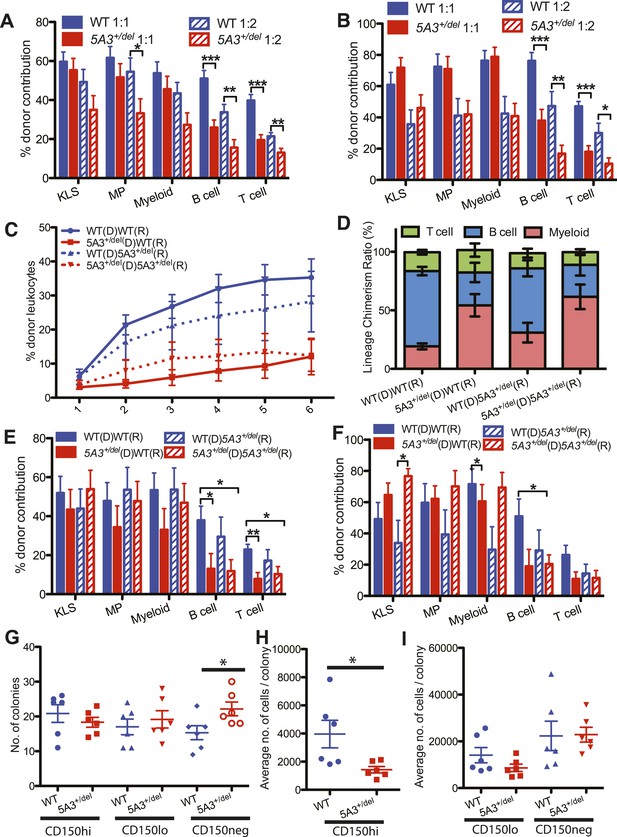
Defective repopulating potential of 5A3+/del BM and CD150hi HSC.
(A, B) BM cells from WT or 5A3+/del mice (n = 9 per genotype) were mixed at ratios of 1:1 and 1:2 with WT competitor cells and transplanted into 2–3 irradiated WT recipients. Percent contribution to the K+L−S+ (KLS), K+L−S− (MP), myeloid, B and T cell lineages in the BM of recipient mice 6 months after primary (A) and secondary (B) transplants are shown. (C) Leukocyte chimerism after competitive transplantation of 15 5A3+/del or WT CD150hi HSC into WT or mutant recipients (n = 12 for WT hematopoietic stem cell (HSC) in WT recipients; n = 12 for 5A3+/del HSC in WT recipients; n = 8 for WT HSC in 5A3+/del recipients; n = 9 for 5A3+/del HSC in 5A3+/del recipients). (D–F) Relative proportions of donor-derived B, T, and myeloid cells in the blood of recipient mice 6 months after transplantation (D). Percent contribution to the K+L−S+ (KLS), K+L−S− (MP), myeloid, B and T cell lineages in the BM of recipient mice 6 months after primary (E) and secondary (F) transplants. Data shown are mean values ±SEM of results from four independent experiments. (G–I) 100 CD150hi HSC, CD150lo HSC, and CD150neg MPP from 8- to 12-week-old 5A3+/del mice and their WT littermates were plated into methylcellulose medium supplemented with cytokines (n = 6 for each genotype). The total number of colonies (G) and the average number of cells per colony (H, I) were assessed after 7 days. Dots represent individual mice, and the horizontal lines indicate median values. Data shown are mean values ±SEM of results from three independent experiments with significant differences between WT and 5A3+/del mice designated by asterisks as follows: *p < 0.05, **p < 0.01, ***p < 0.001.
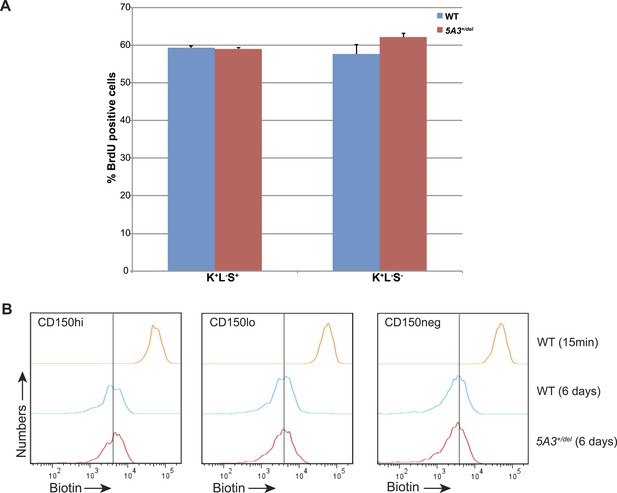
Proliferation and divisional kinetics of 5A3+/del HSCs.
(A) BrdU labeling frequencies of K+L−S+ and K+L−S− cells from WT and 5A3+/del mice taken 24 hr after intraperitoneal injection of BrdU. (B) HSCs were labeled with EZ-Link Sulfo-NHS-LC-LC-biotin (biotin) in vivo, and the in vivo proliferative behavior of HSCs was tracked by dilution of the biotin label caused by cell division. Biotin intensities on CD150hi HSC, CD150lo HSC, and CD150neg MPP taken 15 min and 6 days after intravenous injection of biotin (representative plots from one of 3 mice in each group). Following 6 days of chase, the reduction in biotin label intensity is similar in WT and 5A3+/del CD150hi HSC, CD150lo HSC, and CD150neg MPP.
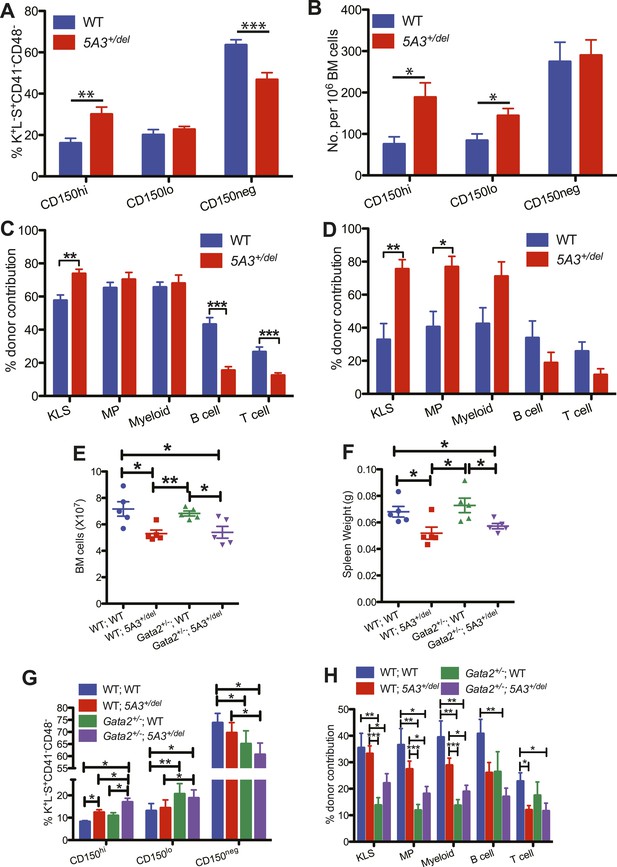
Effects of aging and Gata2 inactivation on 5A3+/del hematopoietic cells.
(A and B) Percent contributions (A) and frequencies (B) of CD150hi HSC, CD150lo HSC, and CD150neg MPP within the K+L−S+CD41−CD48− compartment in 5A3+/del mice (n = 12) and their WT littermates (n = 11) at 24–30 months of age. (C and D) Competitive transplantation of BM cells from 24- to 30-month-old WT or 5A3+/del mice. Percent donor contribution of 5A3+/del cells to K+L−S+ (KLS), K+L−S− (MP), myeloid, B cell, and T cell populations in the BM of recipient mice 4 months after primary (C) and secondary (D) competitive transplantation (n = 5 donors and 10 recipients of each genotype). Data shown are mean values ±SEM of results from two independent experiments. (E) Total numbers of BM cells from 2 femurs and 2 tibiae in 5A3+/del mice, Gata2+/− mice, compound Gata2+/−; 5A3+/del mice and WT littermates at 8–12 weeks of age. (F) Spleen weights in 5A3+/del mice, Gata2+/− mice, compound Gata2+/−; 5A3+/del mice, and WT littermates at 8–12 weeks of age. (G) Percent contributions of cells with high (CD150hi HSC), low (CD150lo HSC), and absent CD150 expression (CD150neg MPP) within the K+L−S+CD41−CD48− compartment of WT, 5A3+/del, Gata2+/−, and compound Gata2+/−; 5A3+/del mice at 8–12 weeks of age (n = 5 of each genotype). (H) BM cells from WT, 5A3+/del, Gata2+/− or compound Gata2+/−; 5A3+/del mice (n = 5 of each genotype) were each mixed at ratios of 1:1 with WT competitor cells and transplanted into two irradiated WT recipients. Percent contribution to the K+L−S+ (KLS), K+L−S− (MP), myeloid, B and T cell lineages in the BM of recipient mice 6 months after primary transplants. Data shown are mean values ±SEM from five independent experiments with significant differences designed by asterisks as follows: *p < 0.05, **p < 0.01, ***p < 0.001. The enhanced repopulating ability of compound Gata2+/−; 5A3+/del vs Gata2 singly mutant HSC achieved borderline statistical significance in three myeloid populations (KLS (p = 0.09), MP (p = 0.09), and myeloid cells (p = 0.12)).
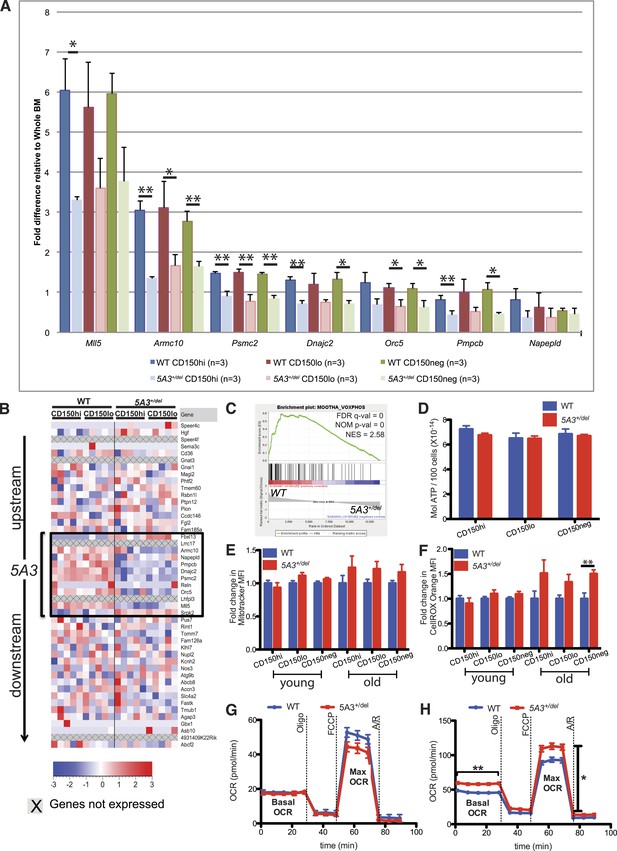
Changes in gene expression and oxidative phosphorylation in 5A3+/del HSC and MP.
(A) Relative mRNA abundances for genes within the deleted 5A3 interval expressed at detectable levels in sorted HSC populations were determined by TaqMan reverse transcriptase PCR (n = 3 per genotype). (B) Expression levels of genes located within and flanking the deleted interval measured by RNA-Seq in sorted CD150hi HSC and CD150lo HSC from 5 mice of each genotype. Each column presents data from an individual mouse, and genes within the 5A3 deleted region are delimited with a black box. Three non-coding RNAs (6030443J06Rik, AC112688.1 and 5031425E22Rik) are located within the 5A3 deletion. Two of these (6030443J06Rik and AC112688.1) are expressed at extremely low levels in HSC, and the other (5031425E22Rik) showed ∼50% lower expression in 5A3+/del HSC. 5031425E22Rik is homologous to the human KMT2E (a.k.a. MLL5) antisense RNA1. Expression levels of the flanking Fbxl13 and Srpk2 genes are modestly up-regulated in 5A3+/del HSC, which is consistent with the targeting strategy used to generate the segmental deletion. (C) Gene Set Enrichment Analysis of 5A3+/del CD150hi HSCs revealed negative enrichment for genes associated with oxidative phosphorylation (OXPHOS). False discovery rate (FDR) q-val, nominal p-value (NOM p-value), and normalized enrichment scores (NESs) are indicated. (D) ATP levels in HSC and MPP from 8- to 12-week-old WT (n = 6) and 5A3+/del (n = 5) mice. Data shown are mean values ±SEM of results from two independent experiments. (E) Fold change in the mean MitoTracker Orange fluorescence levels in 5A3+/del cells normalized to values in WT cells analyzed in the same experiment. (F) Fold change in the mean fluorescence level (MFI) of 5A3+/del cells that are CellROX Orange positive normalized to values in WT cells analyzed in the same experiment. For the MitoTracker and CellROX experiments, n = 13 for WT and n = 12 for 5A3+/del young mice, three independent experiments; n = 5 for WT and n = 6 for 5A3+/del aged mice, two independent experiments. Data shown are mean values ±SEM of results from independent experiments. (G and H) Oxygen consumption rate (OCR) was assessed basally and in response to the mitochondrial inhibitors oligomycin (oligo), carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone (FCCP), and antimycin A and rotenone (A/R) for (G) KLS and (H) MP cells. Data are shown as mean ±SEM of n = 5 mice of each genotype from two independent experiments.





