Auxin-regulated chromatin switch directs acquisition of flower primordium founder fate
Abstract
Reprogramming of cell identities during development frequently requires changes in the chromatin state that need to be restricted to the correct cell populations. Here we identify an auxin hormone-regulated chromatin state switch that directs reprogramming from transit amplifying to primordium founder cell fate in Arabidopsis inflorescences. Upon auxin sensing, the MONOPTEROS transcription factor recruits SWI/SNF chromatin remodeling ATPases to increase accessibility of the DNA for induction of key regulators of flower primordium initiation. In the absence of the hormonal cue, auxin sensitive Aux/IAA proteins bound to MONOPTEROS block recruitment of the SWI/SNF chromatin remodeling ATPases in addition to recruiting a co-repressor/histone deacetylase complex. This simple and elegant hormone-mediated chromatin state switch is ideally suited for iterative flower primordium initiation and orchestrates additional auxin-regulated cell fate transitions. Our findings establish a new paradigm for nuclear response to auxin. They also provide an explanation for how this small molecule can direct diverse plant responses.
https://doi.org/10.7554/eLife.09269.001eLife digest
Plants form new structures such as flowers or branches throughout their life as they develop and grow. However, most plant cells are not able to produce a new flower or branch because the genes involved in these processes are usually switched off. The genes are found in regions of chromatin—the structure in which DNA is packaged in plant cells—that are normally tightly packed. This packing prevents other proteins called transcription factors from accessing the DNA and switching the genes on.
New flowers form from cells that contain high levels of a plant hormone called auxin. In these cells, a protein called MONOPTEROS switches on genes involved in making flowers. How the structure of the chromatin that surrounds these genes is altered so that they can be switched on is not clear. Wu, Yamaguchi, Xiao et al. studied this question in a plant known as Arabidopsis.
The experiments show that MONOPTEROS plays a crucial role in altering the structure of chromatin to allow flowers to form. In the presence of high levels of auxin, MONOPTEROS recruits groups of proteins called SWI/SNF remodeling complexes to regions of chromatin that contain genes involved in flower formation. These protein complexes loosen the structure of the chromatin so that genes can be switched on by transcription factors.
Wu, Yamaguchi, Xiao et al.'s findings suggest that auxin, with the help of MONOPTEROS and the SWI/SNF remodeling complexes, enables flower formation by changing the chromatin state. They further suggest that this chromatin state switch is also involved in leaf formation and other processes in plants that are controlled by MONOPTEROS and auxin.
https://doi.org/10.7554/eLife.09269.002Introduction
Flowers are important for plant reproductive success and for human sustenance. Primordia that give rise to flowers initiate from the organogenic region of the shoot apex that surrounds the central stem cell pool (Smyth et al., 1990). Flower primordium initiation requires a switch from stem cell descendent (transit amplifying cell) to primordium founder cell fate (Barton, 2010). Primordium founder fate is promoted by a local maximum of the hormone auxin and by the AUXIN RESPONSE FACTOR (ARF) MONOPTEROS (MP/ARF5) (Przemeck et al., 1996). In the absence of auxin or MP, shoot apices cannot initiate flower primordia and give rise to characteristic ‘naked pin’ inflorescences (Okada et al., 1991; Przemeck et al., 1996; Vernoux et al., 2000; Cheng et al., 2006). Recently, targets of MP have been identified that promote flower initiation; these include a central regulator of floral fate, LEAFY (LFY), and two regulators of flower growth, AINTEGUMENTA (ANT) and AINTEGUMENTA-LIKE 6 (AIL6) (Cole et al., 2009; Zhao et al., 2010; Yamaguchi et al., 2013; Besnard et al., 2014; Furutani et al., 2014).
Aux/IAA proteins together with co-repressors and repressive chromatin regulators prevent unlicensed auxin response gene expression. In the absence of the auxin stimulus, Aux/IAA proteins associate with the C-terminal domain of MP bound at its target loci (Tiwari et al., 2003; Guilfoyle and Hagen, 2012; Yamaguchi et al., 2013). Aux/IAA proteins directly recruit the transcriptional co-repressor TOPLESS (TPL), which in turn interacts with the histone deacetylase HDA19 (Long et al., 2006; Szemenyei et al., 2008). Histone deacetylation promotes a tight association between histones and the DNA, thus generating a chromatin state refractory to transcription (Eberharter and Becker, 2002). Upon auxin sensing, Aux/IAA proteins are rapidly degraded via the SCFTIR1/AFB ubiquitin ligase, whose substrate recognition F-box module TIR1/AFB binds Aux/IAA proteins in the presence of auxin (Gray et al., 2001; Ramos et al., 2001; Salehin et al., 2015). Aux/IAA degradation leads to dissociation of the co-repressor and HDA19; this is thought to free MP to activate gene expression (Chapman and Estelle, 2009). How MP can execute this important function in the context of the repressive chromatin environment generated by HDA19 is not understood.
Here we uncover a new paradigm for auxin-directed transcriptional and cell fate reprogramming. The reprogramming from transit amplifying to primordium founder cell fate depends on MP-anchored chromatin unlocking by SWI/SNF ATPases. This allows additional transcription factors access to cis regulatory elements previously occluded by nucleosomes. Genetic experiments indicate that SWI/SNF recruitment is an essential function of MP and that SWI/SNF ATPase activity is necessary for reprogramming. Unlicensed chromatin remodeling at MP target loci is prevented by auxin sensitive Aux/IAA proteins, which physically block chromatin remodeler recruitment when complexed with MP. We provide evidence that that the uncovered mechanism underlies additional auxin-controlled cell fate reprogramming events, during embryos patterning and leaf morphogenesis for example.
Results
SWI/SNF ATPases activity is essential for flower primordium initiation
To identify factors that enable auxin-dependent activation of gene expression by overcoming the repressive chromatin at MP target loci, we screened mutants in chromatin regulators for defects in flower primordium initiation. Double hypomorph (brm-3 syd-6) and hypomorph/null (brm-3 syd-5) mutants in two related Arabidopsis SWI/SNF subgroup ATPases BRAHMA (BRM) and SPLAYED (SYD) formed inflorescence ‘pins’ characteristic of auxin pathway mutants (Figure 1A,B). BRM and SYD are both expressed in incipient flower primordia in the inflorescence (Wagner and Meyerowitz, 2002; Wu et al., 2012). brm-1 syd-5 double null mutants are embryonic lethal (Bezhani et al., 2007). To be able to assess the flower primordium initiation in plants that have lost most SYD and BRM activity, we employed the syd-5 null mutant and a conditional BRM mutant, generated by expressing an artificial micro RNA (aMIR) against BRM in adult plants (Wu et al., 2012). aMIRBRM reduces BRM expression in incipient flower primordia (Wu et al., 2012). syd-5 aMIRBRM plants displayed a very dramatic flowerless ‘pin’ phenotype (Figure 1A,B). We next tested whether loss of either BRM or SYD function, neither of which causes a flower primordium initiation defect on its own (Figure 1A,C), enhance the flower initiation defect of the hypomorph mp-S319 allele (Schlereth et al., 2010). Hypomorph mutant phenotypes can be enhanced by loss-of-function in factors that act the same pathway. syd-5 significantly enhanced the primordium initiation defect of the mp-S319 mutant (Figure 1C,D). We could not assess flower primordium initiation in double mutants between the brm-1 null allele and mp-S319 because these plants phenocopied the seedling lethality of the mp-B4149 null mutant (Figure 1—figure supplement 1) (Weijers et al., 2006). However, loss of BRM function in adult plants (aMIRBRM) significantly enhanced the defect in the flower primordium initiation of mp-S319 (Figure 1C,D). The combined data indicate that SWI/SNF ATPase activity is essential for flower primordium initiation.
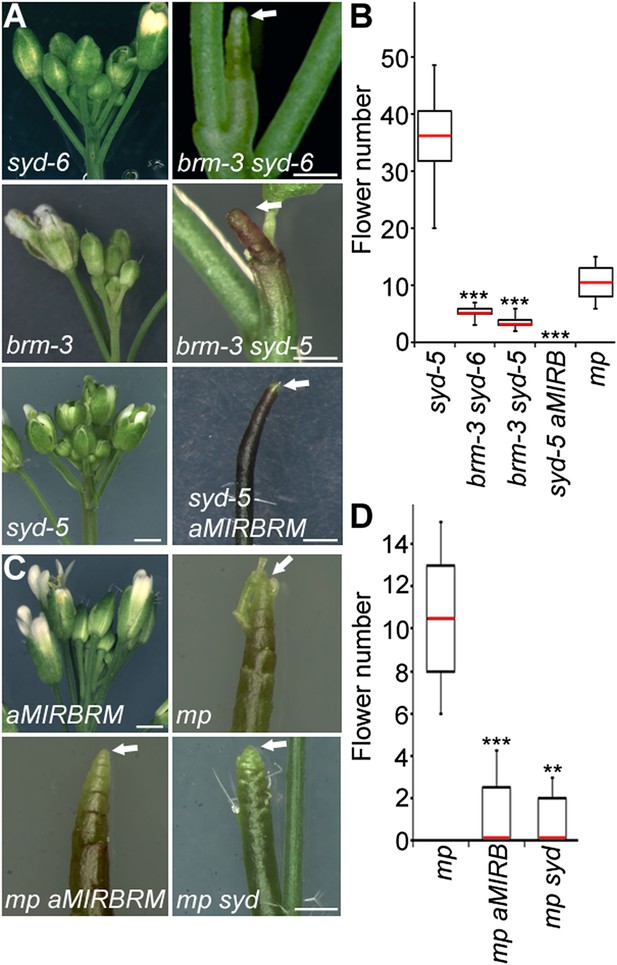
An essential role for SWI/SNF ATPases in flower primordium initiation.
(A) ‘Pin’ inflorescence phenotype (white arrow) of brm syd double mutants. Scale bars = 1 mm. Allelic strength of mutants: syd-6, very weak; brm-3 weak; aMIRBRM, strong; syd-5 null. (B) Quantification of the flower primordia initiated in (A). n > 18. p-value: Mann–Whitney U test. (C) Enhancer tests using hypomorph mp-S319 mutant (Schlereth et al., 2010). White arrows point to ‘pin’ inflorescences. Scale bars = 1 mm. brm-1 null mutant (Hurtado et al., 2006) combined with the mp-S319 hypomorph mutant is seedling lethal like the mp-B4149 null mutant (Weijers et al., 2006) (Figure 1—figure supplement 1) and has developmental defects in the embryo (Figure 1—figure supplement 2). (D) Quantification of the flower primordia initiated in (C). n > 5. p-value: Mann–Whitney U test.
BRM and SYD bind to critical MP targets and are required for their activation
One possible explanation for the striking pin inflorescence phenotype of brm-3 syd-5 double mutants could be that BRM/SYD are required for MP mRNA accumulation in the organogenic region. In situ hybridization did not reveal a visible reduction of MP expression in brm-3 syd-5 shoot apices (Figure 2—figure supplement 1). Alternatively, BRM/SYD may enable MP to activate its target genes. If this were the case, brm-3 syd-5 and mp-S319 should have similar molecular phenotypes. Indeed, expression of the known MP targets LFY and ANT was similarly reduced in mp-S319 and brm-3 syd-5 mutants (Figure 2A). Prior studies suggested that additional MP targets with a role in flower primordium initiation remain unidentified (Yamaguchi et al., 2013). We therefore tested expression of two candidate regulators of flower primordium initiation in mp-S319 and brm-3 syd-5. TARGET OF MONOPTEROS 3 (TMO3) is a direct target of MP during embryo development (Schlereth et al., 2010) that we found to be expressed in the organogenic region of the reproductive shoot apex (Figure 2B). FILAMENTOUS FLOWERS (FIL) encodes a regulator of organ polarity, whose expression changes dramatically during flower initiation (Heisler et al., 2005). Expression of both genes was strongly reduced in mp-S319 and brm-3 syd-5 mutants (Figure 2A) in further support of the idea that BRM/SYD may enable MP target gene activation. The gene expression defects were apparent in the organogenic region of shoot apices just prior to the manifestation of the morphological defect (Figure 2B). To further examine the role of MP in regulation of LFY, ANT, FIL and TMO3 expression, we tested the effect of a steroid inducible gain or loss of MP activity in inflorescences (Yamaguchi et al., 2013). LFY, ANT, FIL and TMO3 accumulation increased shortly after elevating and decreased shortly after reducing MP activity (Figure 2—figure supplement 2).
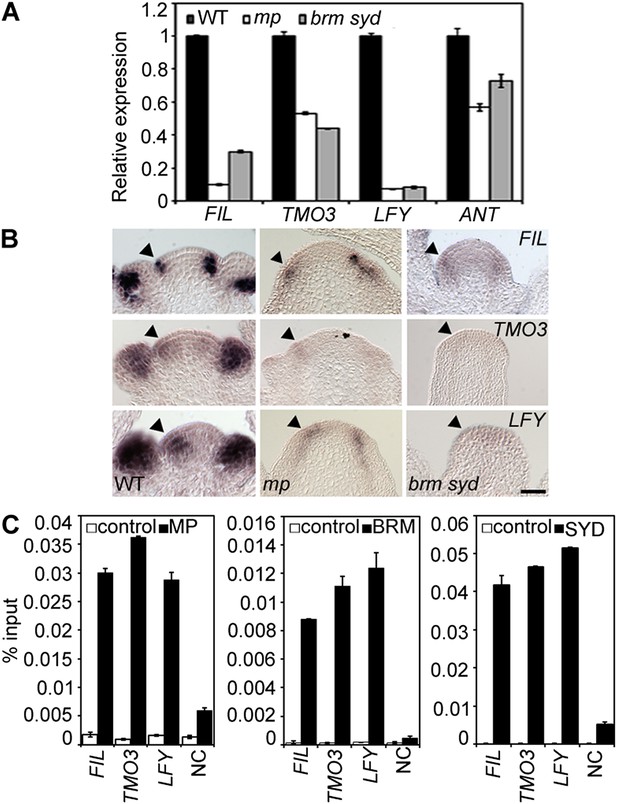
MP and BRM/SYD directly regulate common target genes.
(A) Expression levels of FIL, TMO3, LFY and ANT in wild-type (WT), mp-S319 or brm-3 syd-5 inflorescence shoot apices normalized to that of EIF4A-1. Expression in WT was set to one. (B) In situ hybridization of wild-type, mp-S319 or brm-3 syd-5 inflorescence shoot apices prior to ‘pin’ formation using antisense FIL, TMO3 and LFY probes. Black arrowheads: organogenic region from where flower primordia usually arise. Figure 2—figure supplement 1 shows that MP expression is not visibly reduced in brm-3 syd-5 mutants. Inducible increase or reduction of MP function triggered increased or decreased expression of FIL, TMO3, LFY and ANT, respectively (Figure 2—figure supplement 2). (C) Anti-GFP chromatin immunoprecipitation (ChIP) to test pSYD:GFP-SYD and pBRM:BRM-GFP occupancy at pMP:MP-HA bound sites (as determined by anti-HA ChIP). For MP, BRM and SYD occupancy at the ANT locus see Figure 2—figure supplement 3. For comparison of the binding pattern of BRM, SYD and MP at the FIL, TMO3 or LFY loci see Figure 2—figure supplement 4. Control: anti-GFP or anti-HA ChIP in non-transgenic plants. NC: negative control locus (Ta3 retrotransposon).
On the basis of chromatin immunoprecipitation (ChIP), MP binds to the LFY and ANT loci in inflorescences (Yamaguchi et al., 2013) and to the TMO3 locus in seedlings (Schlereth et al., 2010). We performed MP ChIP to test whether the TMO3 locus was bound in inflorescences and whether MP also associates with the regulatory region of the FIL locus. MP bound both loci in inflorescences (Figure 2C, Figure 2—figure supplement 3, 4). Thus, LFY, FIL, TMO3 and ANT are directly regulated by MP. We next tested, using ChIP, whether BRM and SYD occupy the regulatory regions of these MP target loci. BRM and SYD associated strongly with the LFY, FIL, TMO3 and ANT loci (Figure 2C; Figure 2—figure supplement 3). Finally, we monitored the occupancy of MP, BRM and SYD at different sites throughout the FIL, TMO3 and LFY regulatory regions by ChIP. MP, BRM and SYD exhibited a similar binding pattern at all loci tested (Figure 2—figure supplement 4). We conclude that BRM/SYD and MP occupy similar sites at shared target loci and are required for their transcriptional activation.
FIL contributes to flower primordium initiation
Because FIL expression was dramatically reduced in both mp-S319 and brm-3 syd-5 mutants, we next wished to test whether FIL plays a role in flower initiation. fil-8 null mutants (Goldshmidt et al., 2008) significantly enhanced the mp-S319 hypomorph mutant flower initiation defect (Figure 3A,B). We reasoned the syd-5 null mutants, which show no flower initiation defect on their own due to the redundant role of BRM (Figure 1), should also be enhanced by loss of FIL activity. Indeed, syd fil mutants formed significantly fewer flowers than the parental lines (Figure 3C,D). Higher order mutants in MP targets, such as lfy ant ail-6, form pin inflorescences when treated with a low dose of the auxin transport inhibitor NPA (Yamaguchi et al., 2013). Likewise, lfy fil double mutants formed inflorescence pins when treated with a low dose of NPA (Figure 3E,F). Thus, the direct MP target FIL contributes to initiation of flower primordia.
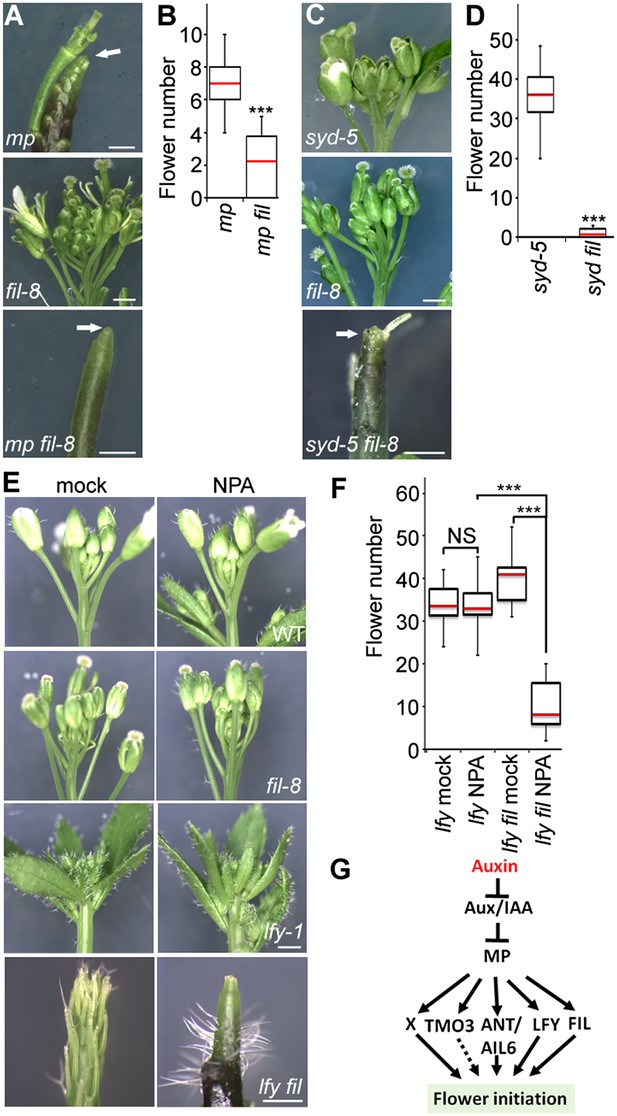
The direct MP and BRM/SYD target FIL plays a role in flower primordium initiation.
(A) Enhancer test using the hypomorph mp-S319 and the null fil-8 mutant. Scale bars = 1 mm. White arrows point to pin inflorescences. (B) Quantification of flower primordia initiated in (A). n > 10. p-value: Mann–Whitney U test. (C) Enhancer test using null syd-5 mutant and fil-8. White arrow points to pin-like inflorescence. Scale bars = 1 mm. (D) Quantification of flower primordia initiated in (C). n > 5. p-value: Mann–Whitney U test. (E) ‘Pin’ inflorescence phenotype of lfy-1 fil-8 double mutant treated with the auxin transport inhibitor N-1-naphthylphthalamic acid (NPA). Scale bars = 1 mm. (F) Quantification of the flower primordia initiated in (E). n > 12. p-value: Mann–Whitney U test. (G) Updated model for auxin/MP-mediated flower primordium initiation together with BRM/SYD. Dashed arrow: role not yet proven. X: additional MP target(s) with a role in flower initiation.
MP physically interacts with and may recruit BRM/SYD
BRM and SYD each are the catalytic subunit of a multiprotein chromatin remodeling complex (reviewed in Han et al., 2015). To test whether MP recruits chromatin remodeling complexes formed around BRM or SYD to its target loci to overcome the repressed chromatin state, we examined whether MP physically interacts with either chromatin remodeling complex. Bimolecular fluorescence complementation (BiFC) and co-immunoprecipitation (co-IP) revealed that MP interacts with the BRM- and the SYD-containing complex (Figure 4A,B) in plant cells. The interaction was enhanced by auxin application (Figure 4B,C; Figure 4—figure supplement 1). No BiFC signal was observed when we used a version of MP that consisted solely of the N-terminal domain (Figure 4A,C; Figure 4—figure supplement 2). Yeast-two-hybrid tests with MP and BRM revealed that no other plant proteins are required for the physical interaction and allowed us to map the interacting region of MP to its middle domain (Figure 4D), which is critical for transcriptional activation (Tiwari et al., 2003). We used the in situ proximity ligation assay (PLA), an immunoassay that allows visualization of protein interactions in tissue sections (Soderberg et al., 2008), to examine where at the shoot apex MP interacts with BRM. On the basis of in situ PLA, MP associates with BRM specifically in the organogenic region of the shoot apex from where flower primordia initiate (Figure 4E). No signal was detected when we performed the PLA assay in plants only expressing pBRM:BRM-GFP (Figure 4E). To directly test whether MP activity is required for BRM and SYD binding to its target loci, we employed ChIP in wild-type and mp-S319 mutant inflorescences. In vivo association of BRM or SYD with the FIL and LFY loci was much reduced in mp-S319 inflorescences (Figure 4F). The data are consistent with the hypothesis that MP may recruit BRM/SYD to target loci.
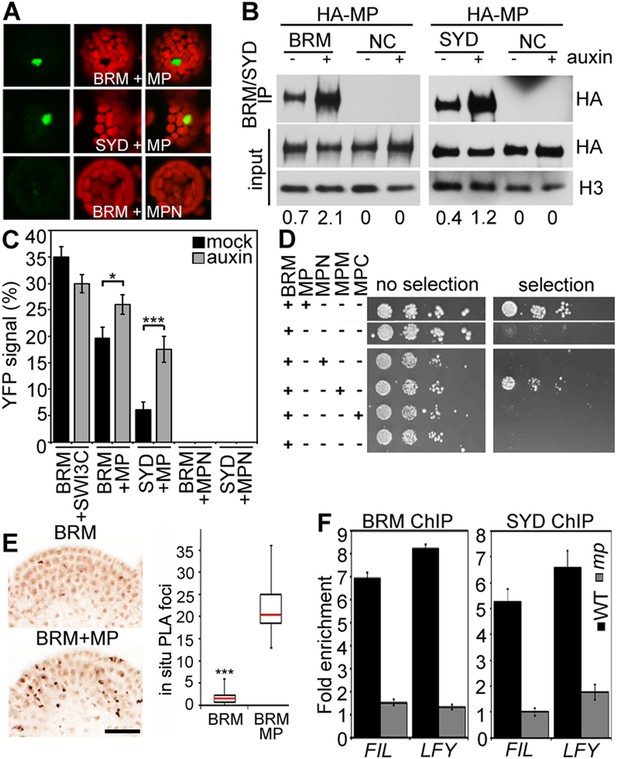
MP physically interacts with and recruits BRM and SYD to target loci.
(A) Bimolecular fluorescence complementation (BiFC) test of MP and BRM or SYD protein interaction in plant cells. Green: BiFC signal in the nucleus, red: chloroplast auto-fluorescence. MPN: N-terminal domain of MP. (B) Co-immunoprecipitation using anti-FLAG antibody in plant cells expressing HA-MP with or without FLAG-BRM or FLAG-SYD. Western blot is probed with anti-HA or anti-histone H3 antibody. Below: Amount of precipitated HA-MP (% of input). See also Figure 4—figure supplement 1. (C) Quantification of BiFC events in the absence or presence of auxin. The error bars are proportional to the standard error of the pooled percentage computed using binomial distribution. n = 3. p-value; Mann–Whitney U test. SWI3C: BRM chromatin remodeling complex component (positive control). (D) Yeast-two-hybrid test of interaction between BRM and MP or MP domains: N: N-terminus, M: middle region, C: C-terminus. See Figure 4—figure supplement 2 for domains of the MP protein. Growth was assayed minus (left) or plus (right) 3-amino-1,2,4-triazole. Thin white line: cropped image from one plate. (E) In situ proximity ligation assay (PLA) with anti-GFP and anti-HA antibodies in pBRM:BRM-GFP or pMP:MP-HA pBRM:BRM-GFP shoot apices. Left: individual sections, right: quantification of interaction foci. n > 12. p-value: Student's t-test. (F) BRM and SYD ChIP enrichment at the FIL and LFY loci relative to the control locus (Ta3 retrotransposon) in wild-type and mp-S319 mutant inflorescences.
SWI/SNF ATPases ‘unlock’ the chromatin for transcriptional activation and flower primordium initiation
Studies in embryos had suggested that MP-interacting Aux/AA proteins recruit the transcriptional co-repressor TPL and the histone deacetylase HDA19 to MP target loci to prevent MP from activating its target genes when auxin levels are low (Long et al., 2006; Szemenyei et al., 2008). We found that TPL and HDA19 occupied the MP-bound sites at the LFY and FIL loci in inflorescence apices in the absence but not in the presence of auxin application, as expected (Figure 5—figure supplement 1A,B). In addition, auxin treatment led to increased histone 3 lysine (acetylation [H3K9ac], an activating histone modification removed by HDA19 [Krogan et al., 2012], at both loci [Figure 5—figure supplement 1C]).
We next tested whether BRM and SYD are required for overcoming the repressed chromatin state generated by TPL and HDA19. BRM or SYD belong to the SWI/SNF subgroup chromatin remodelers, which alter accessibility of the genomic DNA by changing the occupancy or positioning of nucleosomes (Clapier and Cairns, 2009; Han et al., 2012). To assess the accessibility of the MP bound regions at the FIL and LFY loci in inflorescences, we employed Formaldehyde Assisted Isolation of Regulatory Elements (FAIRE), a method that enriches accessible (nucleosome depleted) genomic DNA from crosslinked chromatin after phenol/chloroform extraction (Simon et al., 2012). FAIRE revealed increased accessibility at the FIL and LFY loci after exogenous auxin application (Figure 5A). Likewise, auxin treatment triggered increased H3K9 acetylation at both loci and caused increased FIL and LFY mRNA accumulation (Figure 5B,C). Auxin treatment failed to increase FIL and LFY locus accessibility, presence of activating histone marks, and gene expression in brm-3 syd-5 mutant inflorescences (Figure 5A–C). The combined data suggest that BRM or SYD are necessary for the auxin-dependent increase in accessibility at MP target loci in the context of chromatin, a prerequisite for induction of MP targets.
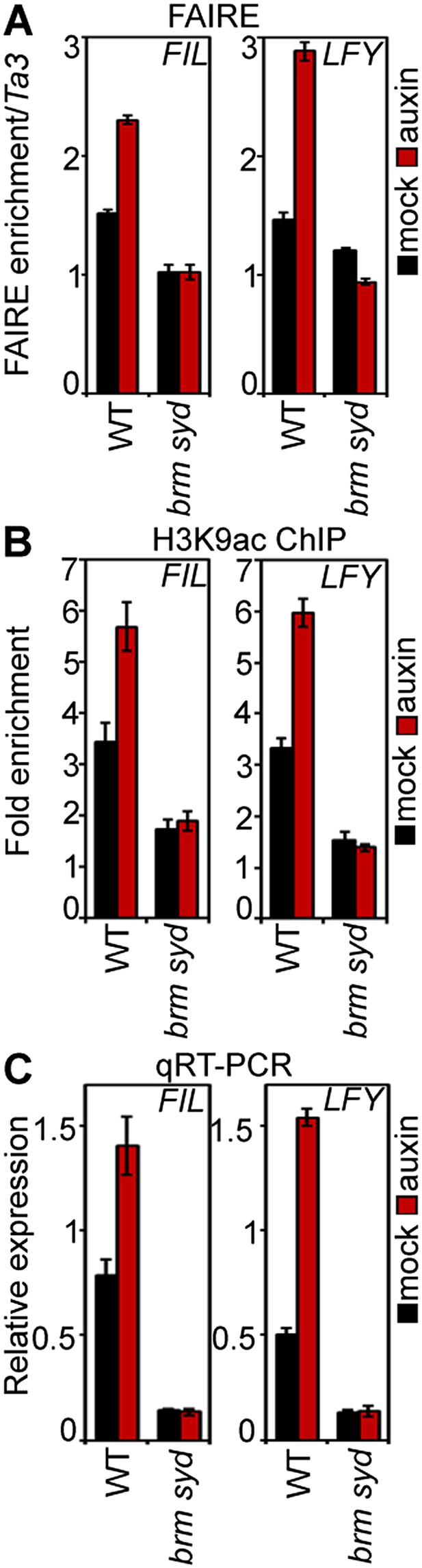
SWI/SNF chromatin remodeling ATPases are required for increased accessibility of MP target loci in response to auxin.
(A) DNA accessibility at FIL and LFY loci in the context of chromatin assayed by Formaldehyde Assisted Isolation of Regulatory Elements (FAIRE) in response to auxin treatment in wild-type (WT) and brm-3 syd-5 inflorescences. The ratio of FAIRE enrichment at the locus of interest was normalized over that at the Ta3 retrotransposon. (B) Anti-histone 3 lysine 9 acetylation (H3K9ac) ChIP at the FIL (left) and LFY (right) locus normalized over that at Ta3 in genotypes and treatments shown in (A). (C) FIL and LFY RNA accumulation relative to EIF4A-1 in genotypes and treatments shown in (A).
SWI/SNF ATPases tethering causes increased accessibility of target loci, transcriptional activation and flower primordium initiation
To test whether BRM/SYD recruitment leads to induction of MP target genes, we tethered the chromatin remodeling complexes to MP targets by fusing the N-terminal DNA binding domain of MP (Tiwari et al., 2003; Boer et al., 2014) to a shared component of the BRM and the SYD chromatin remodeling complex (Han et al., 2015) called BUSHY (MPN-BSH; Figure 6A). MPN-BSH transfection into plant cells caused an increase in endogenous FIL expression (Figure 6B). The magnitude of the response was comparable to that observed upon auxin application (Figure 6B). FIL mRNA levels did not increase when we transfected MPN alone or when we transfected MPN-BSH into brm-3 syd-5 cells, suggesting that the observed activity of the MPN-BSH fusion protein depends on its ability to recruit BRM/SYD (Figure 6B; Figure 6—figure supplement 1). MPN-BSH activity apparently did not require interaction with endogenous MP because introducing a mutation that interferes with homodimerization (Boer et al., 2014) (MPNm1-BSH) did not impair activity (Figure 6—figure supplement 1). By contrast, introducing a second mutation, which abolishes DNA binding specificity, (Boer et al., 2014) (MPNm2-BSH), blocked activity of the fusion protein (Figure 6B). The combined data indicate that BRM/SYD tethering via MPN-BSH is sufficient to induce FIL expression.
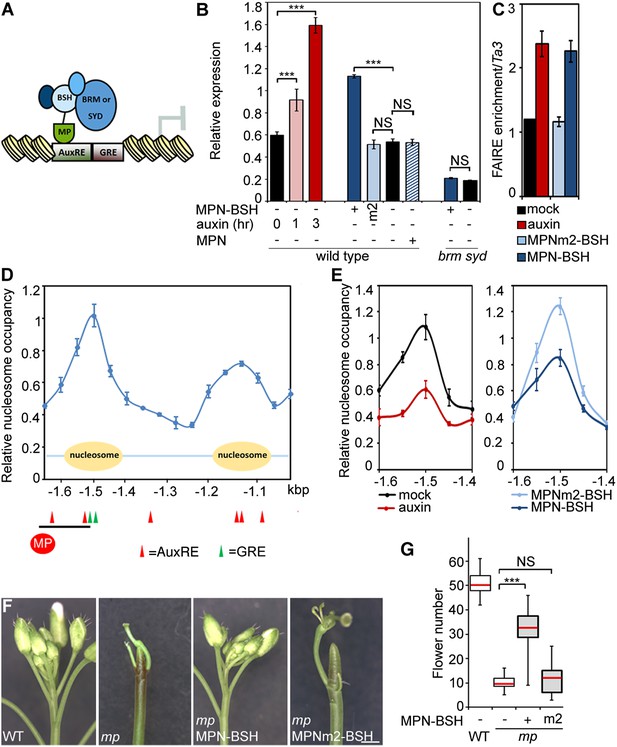
Tethering the BRM or SYD complex to MP target loci mimics MP function.
(A) Tethering of BRM or SYD-containing SWI/SNF complexes to the MP target loci. The shared BRM and SYD complex subunit BUSHY (BSH) (Han et al., 2015) is translationally fused to the MP DNA binding domain (MPN-BSH). (B) Transcriptional activation of the FIL locus by auxin treatment or BRM/SYD tethering via MPN-BSH in isolated plant cells. MPNm1-BSH carries a mutation (G279E; Figure 6—figure supplement 1) that blocks MP dimerization (Boer et al., 2014). MPNm2-BSH carries a second mutation (R215A) that causes loss of DNA binding specificity. Controls: MPN, mock treatment or no plasmid. n > 3. p-value: Student's t-test. (C) DNA accessibility at the FIL locus in response to auxin treatment or BRM/SYD tethering assayed by FAIRE in isolated plant cells. (D) Nucleosome positioning at the FIL locus. Top: MNase digestion followed by tiled oligo qPCR (MNase-qPCR) to monitor nucleosome positioning at the FIL promoter in 3-week-old plants. X-axis: distance from the start codon. Middle: diagram of nucleosome positions. Bottom: red circle: MP protein. Red triangles: core MP binding sites (AuxREs) (Ulmasov et al., 1997; Boer et al., 2014). Black line: region probed in all ChIP or FAIRE experiments (FILb in Figure 2—figure supplement 4). Green triangle: Evolutionarily conserved cis elements. (E) Nucleosome occupancy at the FIL locus in response to auxin treatment or BRM/SYD tethering via MPN-BSH in isolated plant cells by MNase-qPCR. X-axis: distance from the start codon. Figure 6—figure supplement 1 shows the nucleosome occupancy in brm syd mutant plant cells in response to auxin treatment. (F, G) Rescue of mp-S319 flower primordium initiation defect by tethering of BRM or SYD complexes to MP binding sites. Figure 6—figure supplement 2 shows the effect of additional rescue constructs on flower initiation in mp-S319 mutants. Figure 6—figure supplement 3 shows rescue of mp-S319 mutant leaf developmental defects. (F) Representative inflorescence images. Scale bars = 1 mm. (G) Quantification of flower primordium initiation. n > 18. Grey shading: T1 population of transgenic plants. p-value: Mann–Whitney U test.
Next, we monitored the effect of auxin treatment and BRM/SYD tethering on accessibility of the FIL locus. Auxin-treatment or MPN-BSH-transfection caused increased accessibility of the endogenous FIL locus regulatory region on the basis of FAIRE (Figure 6C). We employed limited micrococcal nuclease (MNase) digestion and tiled oligo qPCR to identify a well-positioned nucleosome near the MP and BRM/SYD bound site at the FIL locus (Figure 6D). Auxin treatment or MPN-BSH transfection led to strong destabilization of this nucleosome on the basis of MNase-qPCR in plant cells (Figure 6E). The slightly stronger nucleosome destabilization observed upon auxin treatment was expected; while all cells can respond to auxin, only those transfected (40% on average) can respond to MPN-BSH. Auxin treatment in brm syd mutant cells did not lead to destabilization of the well-positioned nucleosome at the FIL locus (Figure 6—figure supplement 1). We conclude that tethering of BRM or SYD complexes to MP target loci increases their accessibility and transcription.
Finally, we tested whether BRM/SYD tethering can rescue flower primordium initiation in the hypomorph mp-S319 mutant. MPN-BSH and the dimerization defective version MPNm1-BSH caused nearly complete rescue of the flower initiation defects of mp-S319 (Figure 6F,G; Figure 6—figure supplement 2). By contrast, MPNm2-BSH, which has no DNA binding specificity, did not increase flower initiation in mp-S319 mutant plants (Figure 6F,G). Likewise, MPN alone, which cannot recruit BRM/SYD, did not rescue the mp-S319 phenotype (Figure 6—figure supplement 2). The extensive rescue of mp-S319 by MPN-BSH suggests that MP executes its essential role in flower primordium initiation in large part by recruiting BRM and SYD to target loci to ‘open up’ compacted chromatin. That MPN-BSH did not direct ectopic flower initiation in mp-S319 suggests that the auxin pre-pattern is still being correctly interpreted in mp-S319 MPN-BSH, either through the residual MP activity present in mp-S319 (Schlereth et al., 2010), or through other factors. Intriguingly, MPN-BSH and MPNm1-BSH also rescued other phenotypic defects of mp-S319 (Figure 6—figure supplement 3), indicating that SWI/SNF recruitment by MP underlies additional developmental processes controlled by auxin.
An auxin-dependent, MP-anchored, chromatin state switch
Finally, we asked how chromatin remodeler recruitment is limited to cells that have experienced an auxin maximum. Since auxin treatment enhanced MP interaction with both BRM and SYD (Figure 4B,C; Figure 4—figure supplement 1), we hypothesized that auxin-sensitive Aux/IAA proteins might block the interaction between MP and the SWI/SNF ATPases. We probed the effect of two Aux/IAA proteins known to associate with MP, (BODENLOS [BDL] and AUXIN RESISTANT 3 [AXR3]) (Ouellet et al., 2001; Weijers et al., 2006), on the MP interaction with BRM. Presence of either Aux/IAA was sufficient to prevent BRM from associating with MP in yeast (Figure 7A,B). Likewise, auxin-insensitive versions of BDL (bdl) and AXR3 (axr3) strongly interfered with the MP-BRM interaction in plant cells on the basis of co-IP and BiFC experiments (Figure 7C,D). In both yeast and plant assays, only Aux/IAA proteins complexed with MP via the MP C-terminal domain effectively blocked BRM from associating with MP (Figure 7B–D). Finally, increased nuclear accumulation of axr3 (after steroid activation of axr3-GR) caused BRM and SYD dissociation from the LFY and FIL loci (Figure 7E). Thus, Aux/IAA proteins block SWI/SNF ATPase recruitment to MP target loci in the absence of the hormonal cue.
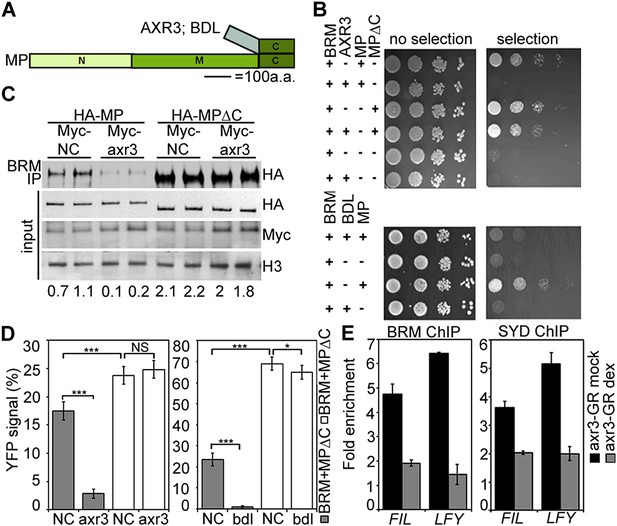
Aux/IAA proteins prevent BRM and SYD recruitment by MP.
(A) Diagram of MP domains. N: N-terminal DNA binding/dimerization domain, M: middle BRM/SYD interacting region, C: C-terminal Aux/IAA interacting domain. (B) Yeast-three-hybrid test of BRM interaction with MP or MP lacking the C-terminal domain (MP∆C) in the presence of the Aux/IAA protein AXR3 (top) or BDL (bottom). Growth was assayed with (right) or without (left) 3-amino-1,2,4-triazole. (C) Co-immunoprecipitation of FLAG-BRM with HA-MP or HA-MP∆C in the presence of the stabilized Myc-axr3. NC: Myc-tagged unrelated protein of similar molecular mass as axr3. Below: Amount of precipitated HA-MP/HA-MP∆C (% input). (D) Quantification of BiFC test of interaction between BRM and MP or BRM and MP∆C in the presence of axr3 (left) or bdl (right) compared to a NC protein. The error bars are proportional to the standard error of the pooled percentage computed using binomial distribution. n = 3. p-value: Mann–Whitney U test. (E) ChIP to assess BRM and SYD association with MP target gene loci before (mock) or after (dex) nuclear entry of axr3-GR. Shown is fold-enrichment relative to a control locus (Ta3 retrotransposon).
Discussion
A molecular framework for acquisition of flower primordium founder fate
A classical role of auxin is initiation of flower primordia from the organogenic region of the shoot apex. Flowers are critical for plant reproductive success and human sustenance. Despite its importance, mechanistic insight into the nuclear responses that underlie auxin-mediated cell fate reprogramming is lacking. We show here that after perception of the hormonal cue, the ARF MP executes its central role (Przemeck et al., 1996; Reinhardt et al., 2003) in flower primordium initiation in large part by recruiting BRM or SYD-containing chromatin remodeling complexes to its target loci to unlock compacted chromatin. Tethering SWI/SNF complexes to MP target loci led to extensive genetic rescue of mp-S319 mutant flower initiation defects, while loss-of-function analyses uncovered an essential role for the chromatin remodeling ATPases in induction of MP target genes and in flower primordium initiation. Unlicensed activation of MP targets in the absence of the hormonal stimulus is prevented by MP interacting Aux/IAA proteins, which noncompetitively inhibit BRM or SYD complex recruitment (Figures 7 and 8). This prevents premature overturning of the repressive chromatin state generated by the TPL/HDA19 complex (Long et al., 2006; Szemenyei et al., 2008).
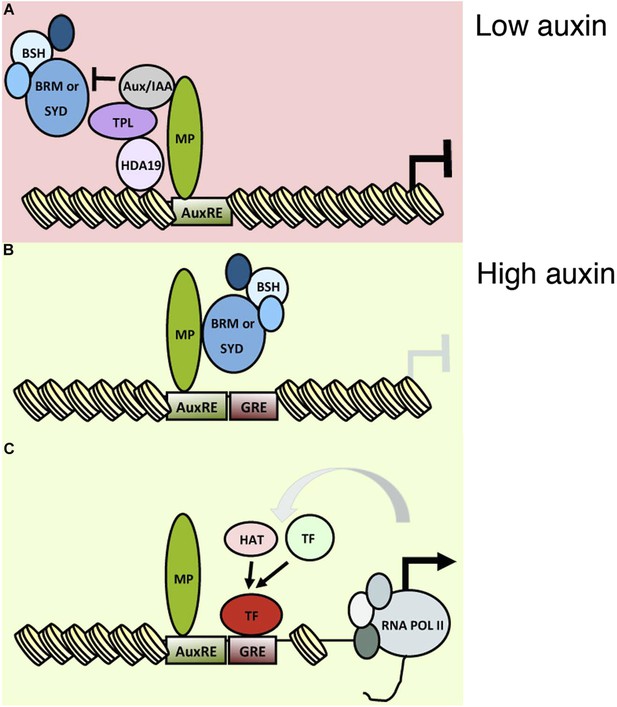
An auxin triggered chromatin state switch.
(A) In conditions of low auxin, Aux/IAA proteins bind to MP transcription factor associated with target loci and prevent gene expression in two ways: by recruiting the co-repressor TOPLESS (TPL) and histone deacetylase HDA19 and by preventing recruitment of the BRM or SYD chromatin remodeling complexes. (B) Upon establishment of a local auxin maximum, Aux/IAA proteins are degraded, this leads to eviction of HDA19 and TPL. Aux/IAA degradation also frees MP to recruit BRM or SYD complexes. The chromatin remodeling complexes open up the compacted chromatin by reducing nucleosome occupancy, thus increasing the accessibility of the genomic DNA near MP bound sites. (C) The ‘chromatin unlocking’ allows additional transcription factors access to their cis elements. This, possibly via additional steps, leads to recruitment of the general transcriptional machinery and initiation of transcription. HAT: histone acetyl transferase. GRE: binding site for transcription factor (TF). Figure 8—figure supplement 1 shows evolutionarily conserved cis elements near the midpoint of the well-positioned nucleosome at the FIL locus.
A versatile, auxin tunable chromatin state switch for cell identity reprogramming
Our study uncovers a rapid, and precise MP-anchored chromatin state switch that underlies flower primordium initiation. These attributes make it well suited to support both iterative initiation of floral primordia as new local auxin maxima form and positioning of flower primordia at the correct phyllotactic distance from one another (Heisler et al., 2005; Traas, 2013). It is rapid because Aux/IAA proteolysis is triggered immediately upon auxin sensing (Gray et al., 2001; Ramos et al., 2001), this allows a rapid onset of the ‘ON’ state. In addition, BRM and SYD complexes are present in all rapidly dividing cells (Wagner and Meyerowitz, 2002; Bezhani et al., 2007; Wu et al., 2012). It is precise due to the inherent auxin sensitivity of individual Aux/IAA proteins (Calderón Villalobos et al., 2012; Havens et al., 2012), this enables tuning of the switch to specific auxin thresholds.
BRM and SYD act as ‘gatekeepers’ for auxin-triggered transcriptional activation. Subsequent to chromatin remodeling, additional sequence specific binding proteins gain access to their previously occluded binding sites. The well-positioned nucleosome near the MP-bound site at the FIL locus, which is destabilized by auxin treatment or SWI/SNF tethering, is positioned over evolutionary conserved cis elements (Figure 8—figure supplement 1), some of which had previously been shown to co-occur with AuxREs (Berendzen et al., 2012). The transcription factors that can gain access to their binding sites only after chromatin remodeling allow additional layers of selectivity. For example, different subsets of the MP and SWI/SNF unlocked genes may be activated in different cell types based on the prevailing transcription factor repertoire. In addition, this mechanism supports a protracted response (Mahonen et al., 2014), if the accumulation of critical transcription factors is delayed relative to chromatin unlocking. The uncovered paradigm for auxin-triggered transcriptional activation thus helps explain how auxin can direct many different plant responses.
A general and conserved framework for auxin controlled cell fate reprogramming
The phytohormone auxin is a key signal in plant morphogenesis, controlling most aspects of plant development and growth (Reinhardt et al., 2000; Scarpella et al., 2010; Domagalska and Leyser, 2011; Salehin et al., 2015; ten Hove et al., 2015). Our genetic enhancer and rescue tests implicate chromatin remodeling by BRM and SYD in embryogenesis, root development, seedling viability and leaf development (Figure 1—figure supplement 1; Figure 1—figure supplement 2; Figure 6—figure supplement 3). This suggests that MP-dependent recruitment of BRM/SYD and chromatin ‘unlocking’ is required for many developmental processes controlled by auxin and may be a general mechanism for auxin-triggered cell fate reprogramming. Other activating ARFs (Tiwari et al., 2003) may also recruit BRM/SYD. All components required for regulatory switch—from the SCFTIR1/ABF complex to ARF, Aux/IAA, BRM/SYD, TPL, HDAC—are conserved in all land plants (Remington et al., 2004; De Smet et al., 2011; Sang et al., 2012; Kato et al., 2015), suggesting the possibility that it represents an ancient regulatory module.
Reprogramming of cell identities during development frequently requires chromatin state changes (Chen and Dent, 2014). A key question is how general chromatin remodelers can function in a specific genomic context to change the fate of a restricted cell population in a precise, cue dependent manner (Han et al., 2015). Here, we uncover a simple and elegant mechanism for small-signaling-molecule-regulated chromatin state switch that is anchored to precise genomic locations by a master transcription factor, can rapidly respond to a range of signaling molecule concentrations and is versatile in that it supports diverse transcriptional and cell fate identity outcomes.
Materials and methods
Plant materials and treatments
Request a detailed protocolMutant alleles and transgenic plants used in this study include brm-1 (Hurtado et al., 2006), brm-3 (Farrona et al., 2007), syd-5 (Bezhani et al., 2007), syd-6 (Han et al., 2012), pLFY:aMIRBRM (Wu et al., 2012), mp-S319 (Cole et al., 2009), mp-B4149 (Weijers et al., 2006), arf7-1 (Okushima et al., 2005), ap1-1 cal-1 (Ferrandiz et al., 2000), fil-8 (Goldshmidt et al., 2008), lfy-1 (Weigel et al., 1992), pBRM:BRM-GFP and pSYD:GFP-SYD (Wu et al., 2012), 35S:TPL-GFP (Long et al., 2006). All are in the Columbia accession. Flower number was counted at 60 to 65 DAG (days after germination). For expression and ChIP, 5 cm bolt inflorescences were treated with 10 µM dexamethasone (DEX, Sigma St. Louis MO, United States) or 10 µM indole-3-acetic acid (IAA, Sigma) plus 0.015% Silwet-77. For mock treatments, 0.1% ethanol or 0.1% DMSO plus 0.015% Silwet-77 were used. Inflorescences were harvested 6 hr after treatment. For auxin treatments in protoplasts, 4 × 106 cells were harvested from leaves of 15 day-old long-day-grown plants and treated with 10 µM IAA in 0.02% ethanol in buffer W5 (Yoo et al., 2007). Treatment duration ranged from 15 min to 3 hr. For 1-N-Naphthylphthalamic acid (NPA, Sigma) treatments, 15-day-old seedlings were sprayed with 10 nM NPA plus 0.015% Silwet-77 or with 0.1% DMSO plus 0.015% Silwet-77 every 3 days for total of 9 treatments. To test for gene expression changes upon inducible increase or reduction in MP activity, 14-day old long-day grown seedlings of plants expressing and estradiol inducible version of MP (pER>>MP∆C) or a dexamethasone inducible version or AXR3 (ap1cal axr3-GR) were sprayed with 10 μM β-estradiol (Sigma) in 0.05% ethanol or 10 µM dexamethasone (Sigma) in 0.05% ethanol. Mock treatment was with 0.05% ethanol. Samples were harvested at 3 hr or 6 hr after treatment.
Transgenic plants
Request a detailed protocolTo generate pMP:MP-6xHA, a full-length MP genomic fragment was cloned into pENTR/D-TOPO (Thermo Fisher, Waltham MA, United States). The stop codon was replaced by a SpeI site and a 6xHA tag was inserted. The pMP:MP-6xHA clone was shuttled into pKGW (Karimi et al., 2002). To generate axr3-GR, the stop codon of axr3 in pKGW was replaced by an NdeI site and the rat glucocorticoid receptor was inserted at the 3′ end of axr3. The 35S promoter was cloned into pKGW to obtain 35S:axr3-GR by LR clonase. To generate estradiol inducible MP, a truncated version of MP was missing the C-terminal PB1 domain (amino acids 795–902) was amplified from cDNA, cloned into pENTR/D-TOPO (Thermo Fisher) and sequence verified. The clone was shuttled into the estradiol-inducible expression vector pMDC7 (Curtis and Grossniklaus, 2003). To generate HDA19-GFP, a HDA19 genomic fragment was amplified and cloned into pENTR/D-TOPO. The stop codon was replaced by a SpeI site and the green fluorescence protein (GFP) coding region was inserted. The pHDA19:HDA19-GFP fragment was cloned into the NotI site of the binary vector pMLBART. To generate 35S:MPN-BSH, 35S:MPNm1-BSH and 35S:MPNm2-BSH, the N-terminal MP DNA binding domain (MPN, [amino acids 1–348]), a dimerization mutant (MPNm1, G279E, [Boer et al., 2014]), or a dimerization and DNA binding mutant (MPNm2, G279E R215A, [Boer et al., 2014]), were fused in-frame with the full length coding region of BSH (Bezhani et al., 2007) and sub-cloned into pUC19. The resulting MPN-BSH cDNAs were cloned into pGWB2 (Nakagawa et al., 2007). For 35S:MPN and 35S:MPN-BSH, the MPN and MPNm1 fragments were cloned into pENTR/D-TOPO and recombined into pGWB2 (Nakagawa et al., 2007). All constructs were transformed into mp-S319/+ plants by floral dip. For primer sequences see Supplemental file 1.
Expression analysis
Request a detailed protocolqRT-PCR was performed as previously described (Yamaguchi et al., 2013). Expression levels were determined by real-time PCR and normalized over that of EUKARYOTIC TRANSLATION INITIATION FACTOR 4A-1 (EIF4A-1; At3g13920). The mean and standard error were determined using three technical replicates from one representative biological replicate. Two to three biological replicates were performed. The LFY and MP probes for in situ hybridization have been described (Yamaguchi et al., 2013). FIL, and TMO3 probes were amplified and cloned into pGEM-T (Promega, Fitchburg WI, United States). RNA in situ hybridization was performed as previously described (Wu and Wagner, 2012). Inflorescences were harvested and fixed at 24 DAG, before manifestation of the pin inflorescence phenotypes in mp-S319 and brm-3 syd-5. Sections to be directly compared were processed together on the same slide. Protoplasts were transfected as described (Yoo et al., 2007). After transfection, or after auxin or mock treatment, protoplasts were harvested; RNA was extracted using the RNeasy Micro kit (Qiagen). cDNA was synthesized from 100 ng total RNA using the superscript III kit (Thermo Fisher). For gene expression analysis in pER>>MP∆C or ap1 cal axr3-GR, 4 µg of total RNA was used for reverse transcription with the superscript IV kit (Thermo Fisher).
ChIP
Request a detailed protocolChIP was performed as previously described (Yamaguchi et al., 2014). The following antibodies were used: anti-GFP (A6455, Thermo Fisher), anti-HA (12CA5, Roche, Basel, Switzerland), anti-Histone H3K9ac antibody (39138, Active Motif, Carlsbad CA, United States) and anti-SYD (Wagner and Meyerowitz, 2002). Two to three biological replicates were performed for each ChIP experiment. The Ta3 retrotransposon (At1g37110) was used as the negative control (NC) locus for all ChIP experiments. Nontransgenic plants of the same age served as ChIP control genotypes. When comparing binding in different genotypes (wild type vs mutant), percent input enrichment in each ChIP sample was normalized over that at the NC locus to compute fold enrichment. To enrich for incipient flower primordia, ChIP experiments displayed in Figure 2 and Figure 2—figure supplement 2 were performed in the ap1-1 cal-1 (Ferrandiz et al., 2000) genetic background. For primer sequences see Supplemental file 1.
FAIRE
Request a detailed protocolFAIRE was performed as described (Omidbakhshfard et al., 2014). For inflorescences, 0.3 g of tissue was crosslinked with 1% formaldehyde in crosslinking buffer under vacuum for 8 min. For tests in plant cells, 1 × 106 protoplasts were crosslinked in 1% formaldehyde, 1x PBS for 8 min. Isolated DNA fragments were further purified by Qiaquick DNA purification columns (Qiagen, Germantown MD, United States). The Ta3 retrotransposon (At1g37110) (Johnson et al., 2002) was used as the NC locus for all FAIRE experiments. qPCR was performed for crosslinked and noncrosslinked FAIRE samples. Fold enrichment was obtained by normalizing DNA accessibility in FAIRE samples over that of un-crosslinked DNA. The fold enrichment at each experimental locus was normalized over that of Ta3.
Protein interaction
Request a detailed protocolTo test for interaction between BRM and MP in yeast, full-length MP (amino acids 1–902), MPN (amino acids 1–348), MPM (amino acids 349–765) and MPC (amino acids 766–902) (Tiwari et al., 2003) were cloned into pDEST22 (Thermo Fisher). The N-terminal protein interaction domain of BRM (amino acids 1–976) (Wu et al., 2012; Efroni et al., 2013) was used as bait. The pDEST22 MP constructs and pDEST32 BRM were co-transformed into yeast strain AH109 (Clontech, Mountainview CA, United States). For yeast-three-hybrid analyses, BRM (amino acids 1–976) was fused to the GAL4 DNA binding domain in pBridge (Clontech). Full-length AXR3 or BDL were cloned behind the MET25 promoter into the same vector. Full-length MP and MP∆C (amino acids 1–765) were cloned into pACT2 (Clontech). Constructs in pBridge and pACT2 were cotransformed into yeast strain AH109. Serial dilutions of transformed cells grown for 72 hr on -Trp-Leu (-Met) and on -Trp-Leu (-Met)-His/SD medium with 0.5 to 0.1 mM 3-amino-1,2,4-triazole (Y2H and Y3H, respectively).
For bimolecular fluorescence complementation (BiFC), the above mentioned fragments of BRM and MP and amino acids 1 to 657 of SYD (Wu et al., 2012), were shuffled into pSPYNE(R)173 and pSPYCE(MR) (Waadt et al., 2008). 4xMyc-axr3 and 4xMyc-bdl in pUC19 were used for BiFC competition assays. BiFC in protoplasts was performed as previously described (Yoo et al., 2007). For each experiment, YFP signal was compared only within protoplast populations prepared and transformed at the same time. Images were taken with a confocal microscope with the same gain (Leica, LCS SL). Multiple images were taken for each biological replicate. The interaction frequency was calculated by counting the number of YFP positive nuclei among all protoplasts under an epifluorescence microscope (Olympus, MVX100). At least one hundred and fifty protoplast cells were counted for each sample; three biological replicate samples were performed for each combination tested.
For co-immunoprecipitation assays, FLAG-BRM plus 3xHA-MP/MPN, FLAG-SYD plus 3xHA-MP/MPN or FLAG-BRM plus 3xHA-MP/MP∆C plus 4xMyc-AXR3/BDL/PI cloned into pUC19 were co-transfected into Arabidopsis leaf protoplasts. PI (PISTILLATA) served as NC protein in the competitions because of its similar molecular mass to AXR3 and BDL. The nuclear fraction of the protoplasts was prepared and co-immunoprecipitation was conducted essentially as previously described (Ryu et al., 2007). Anti-FLAG (1:2000; 9A3, Cell Signaling, Danvers MA, United States) was used for immunoprecipitation. Anti-HA-peroxidase high affinity (1:1000; 3F10, Roche), anti-c-Myc (1:2000; C3956, Sigma), or anti-H3 (1:5000, ab1791, AbCam, Cambridge MA, United States) were used for Western blotting. Band signal intensity was quantified using image J (Schneider et al., 2012). The signal intensity of immunoprecipitated HA-MP was normalized over that of HA-MP in the input for each sample to obtain percent input enrichment.
For in situ proximity ligation assays (PLA), inflorescences (3 cm bolt) were fixed in 4% paraformaldehyde, 1 × PBS, 0.1% Triton X-100 overnight at 4°C. Inflorescences were dehydrated, embedded and sectioned as for in situ hybridization (Wu et al., 2012). The antigen was unmasked by heat-induced antigen retrieval in 10 mM Tris–HCl and 1 mM EDTA (pH 9) for 40 min. Rabbit anti-GFP (1:1600; 2555, Cell Signaling) and mouse anti-HA (1:1200; 6E2, 2367, Cell Signaling) antibodies were applied to sections and incubated overnight at 4°C. PLA was performed according to manufacturer's instructions (Duolink, Sigma) with the following modifications: sections were incubated with PLUS and MINUS PLA probes overnight at 4°C, ligation was performed at 37°C for 2 hr and amplification was performed at 37°C for 3 hr. Rolling-circle products were visualized with horseradish peroxidase (HRP)-labeled probes (Duolink in situ Detection Reagents Brightfield, Sigma). The number of rolling circle products was counted under a brightfield microscope (Olympus, BX51).
Micrococcal nuclease (MNase) digestion
Request a detailed protocol2 g of above ground tissue was harvested without crosslinking and nuclei and chromatin were isolated as previously described (Chodavarapu et al., 2010) with minor changes. The nuclear pellet was washed twice with HBB buffer. The isolated chromatin was digested with a final concentration of 0.2–0.5 units/μl MNase (Takara, Tokyo, Japan) for 3 min in digestion buffer at 37°C. Subsequent steps were performed as previously described (Chodavarapu et al., 2010). Relative nucleosome occupancy was analyzed by tiled oligo qPCR. Percent input enrichment for each primer pair was extrapolated using a dilution series of undigested genomic DNA (Gévry et al., 2009). Fold enrichment of nucleosome bound DNA was calculated by normalizing percent input of each primer pair over that of the gypsy-like retrotransposon (At4g07700). MNase in protoplasts was performed as in intact tissues with some modification. 2 × 106 cells were harvest by centrifugation at 11,800 rpm for 2 min, followed by resuspension in 500 µl lysis buffer by vortexing. After centrifugation at 7300 rpm for 5 min, the nuclear fraction was resuspended in HBC buffer (Chodavarapu et al., 2010). The chromatin was digested with a final concentration of 0.02 units/μl MNase (Takara). For primer sequences see Supplemental file 1.
Data analysis and presentation
Request a detailed protocolMean ± SEM is shown for all numerical values, for frequencies the error bars are proportional to the standard error of the pooled percentage computed using binomial distribution . For qRT-PCR and ChIP one representative of three experiments is shown. For all other data normal distribution was tested by the Kolmogorov–Smirnov test. For normally distributed data, statistical significance was computed using a two-tailed Student's t-test. For non-normally distributed data, statistical significance was computed using a two-tailed Mann–Whitney U test. Significance cutoff (*) p < 0.01. NS = Not significant. **p < 0.001, ***p < 0.001. Box and whisker plots: lower vertical bar: sample minimum. Lower box: lower quartile. Red line: median. Upper box: upper quartile. Upper vertical bar: sample maximum. For flower initiation tests, the parental line with the fewest flowers served as control.
References
-
A combinatorial TIR1/AFB-Aux/IAA co-receptor system for differential sensing of auxinNature Chemical Biology 8:477–485.https://doi.org/10.1038/nchembio.926
-
Mechanism of auxin-regulated gene expression in plantsAnnual Review of Genetics 43:265–285.https://doi.org/10.1146/annurev-genet-102108-134148
-
Chromatin modifiers and remodellers: regulators of cellular differentiationNature Reviews Genetics 15:93–106.https://doi.org/10.1038/nrg3607
-
The biology of chromatin remodeling complexesAnnual Review of Biochemistry 78:273–304.https://doi.org/10.1146/annurev.biochem.77.062706.153223
-
Unraveling the evolution of auxin signalingPlant Physiology 155:209–221.https://doi.org/10.1104/pp.110.168161
-
Signal integration in the control of shoot branchingNature reviews. Molecular Cell Biology 12:211–221.https://doi.org/10.1038/nrm3088
-
A nucleosome interaction module is required for normal function of Arabidopsis thaliana BRAHMAJournal of Molecular Biology 373:240–250.https://doi.org/10.1016/j.jmb.2007.07.012
-
Redundant regulation of meristem identity and plant architecture by FRUITFULL, APETALA1 and CAULIFLOWERDevelopment 127:725–734.
-
MAB4-induced auxin sink generates local auxin gradients in Arabidopsis organ formationProceedings of the National Academy of Sciences of the USA 111:1198–1203.https://doi.org/10.1073/pnas.1316109111
-
Nucleosome mappingMethods in Molecular biology 543:281–291.https://doi.org/10.1007/978-1-60327-015-1_19
-
Roles and activities of chromatin remodeling ATPases in plantsThe Plant Journal 83:62–77.https://doi.org/10.1111/tpj.12877
-
A synthetic approach reveals extensive tunability of auxin signalingPlant Physiology 160:135–142.https://doi.org/10.1104/pp.112.202184
-
GATEWAY vectors for Agrobacterium-mediated plant transformationTrends in Plant Science 7:193–195.https://doi.org/10.1016/S1360-1385(02)02251-3
-
Molecular basis for AUXIN RESPONSE FACTOR protein interaction and the control of auxin response repressionProceedings of the National Academy of Sciences of the USA 111:5427–5432.https://doi.org/10.1073/pnas.1400074111
-
Development of series of gateway binary vectors, pGWBs, for realizing efficient construction of fusion genes for plant transformationJournal of Bioscience and Bioengineering 104:34–41.https://doi.org/10.1263/jbb.104.34
-
A step-by-step protocol for formaldehyde-assisted isolation of regulatory elements from Arabidopsis thalianaJournal of Integrative Plant Biology 56:527–538.https://doi.org/10.1111/jipb.12151
-
IAA17/AXR3: biochemical insight into an auxin mutant phenotypeThe Plant Cell 13:829–841.https://doi.org/10.1105/tpc.13.4.829
-
Contrasting modes of diversification in the Aux/IAA and ARF gene familiesPlant Physiology 135:1738–1752.https://doi.org/10.1104/pp.104.039669
-
Control of leaf and vein development by auxinCold Spring Harbor Perspectives in Biology 2:a001511.https://doi.org/10.1101/cshperspect.a001511
-
NIH Image to ImageJ: 25 years of image analysisNature Methods 9:671–675.https://doi.org/10.1038/nmeth.2089
-
PIN-FORMED 1 regulates cell fate at the periphery of the shoot apical meristemDevelopment 127:5157–5165.
-
SWI2/SNF2 chromatin remodeling ATPases overcome polycomb repression and control floral organ identity with the LEAFY and SEPALLATA3 transcription factorsProceedings of the National Academy of Sciences of the USA 109:3576–3581.https://doi.org/10.1073/pnas.1113409109
-
RNA in situ hybridization in ArabidopsisMethods in Molecular biology 883:75–86.https://doi.org/10.1007/978-1-61779-839-9_5
-
PROTOCOLS: Chromatin Immunoprecipitation from Arabidopsis TissuesArabidopsis Book 12:e0170.https://doi.org/10.1199/tab.0170
-
A molecular framework for auxin-mediated initiation of flower primordiaDevelopmental Cell 24:271–282.https://doi.org/10.1016/j.devcel.2012.12.017
-
Hormonal control of the shoot stem-cell nicheNature 465:1089–1092.https://doi.org/10.1038/nature09126
Article and author information
Author details
Funding
National Science Foundation (NSF) (IOS grant 1257111)
- Doris Wagner
National Science Foundation (MCB-0925071)
- Doris Wagner
Japan Society for the Promotion of Science (JSPS) (Postdoctoral Fellowship for Research Abroad)
- Nobutoshi Yamaguchi
Howard Hughes Medical Institute (HHMI)
- Mark Estelle
Gordon and Betty Moore Foundation
- Mark Estelle
National Institutes of Health (NIH) (GM43644)
- Mark Estelle
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Acknowledgements
We thank J Alonso, M Bayer, JD Wagner, D Weijers and Z Spiegelman for comments, J Kim for advice on statistical analyses and D Weijers and J Reed for materials (mp-B4149 seed and axr3 clone, respectively). This work was supported by NSF IOS grant 1257111 and MCB-0925071 to DW, by a Japan Society for the Promotion of Science Postdoctoral Fellowship for Research Abroad to NY and by Howard Hughes Medical Institute, Gordon and Betty Moore Foundation, and the NIH (GM43644) to M.E.
Copyright
© 2015, Wu et al.
This article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use and redistribution provided that the original author and source are credited.
Metrics
-
- 7,083
- views
-
- 1,687
- downloads
-
- 216
- citations
Views, downloads and citations are aggregated across all versions of this paper published by eLife.
Citations by DOI
-
- 216
- citations for umbrella DOI https://doi.org/10.7554/eLife.09269





