Protein kinase C coordinates histone H3 phosphorylation and acetylation
Figures
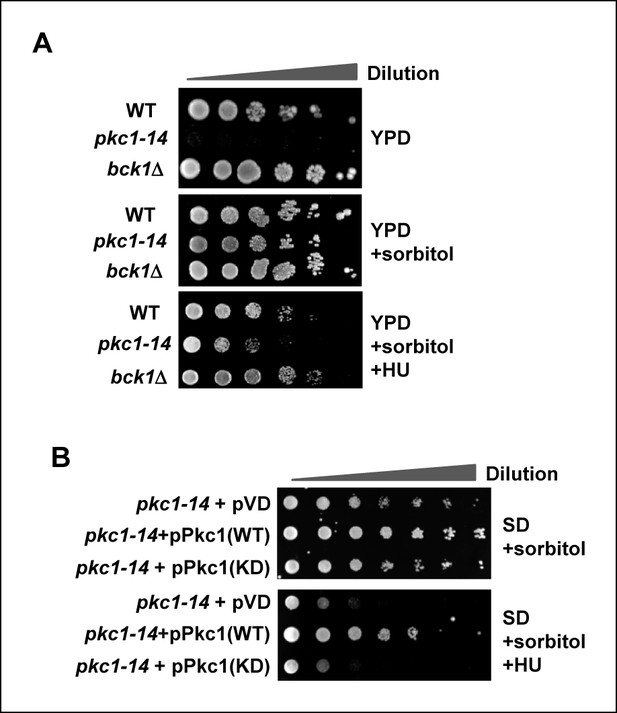
Pkc1p is required for cell viability and chromatin integrity in the presence of hydroxyurea.
(A and B) Cell growth assays. (A) 10 fold serial dilutions of wild-type (WT) (DK186), pkc1-14 (DK1690) or bck1Δ (Y01328) cells were plated onto YPD media in the presence or absence of 1M sorbitol or (B) pkc1-14 (DK1690) cells containing empty vector or plasmids expressing WT or kinase dead (KD) Pkc1, were plated onto SD media in the presence 1M sorbitol. Cells were grown at 34ºC and where indicated, 100 mM HU was added to the media.
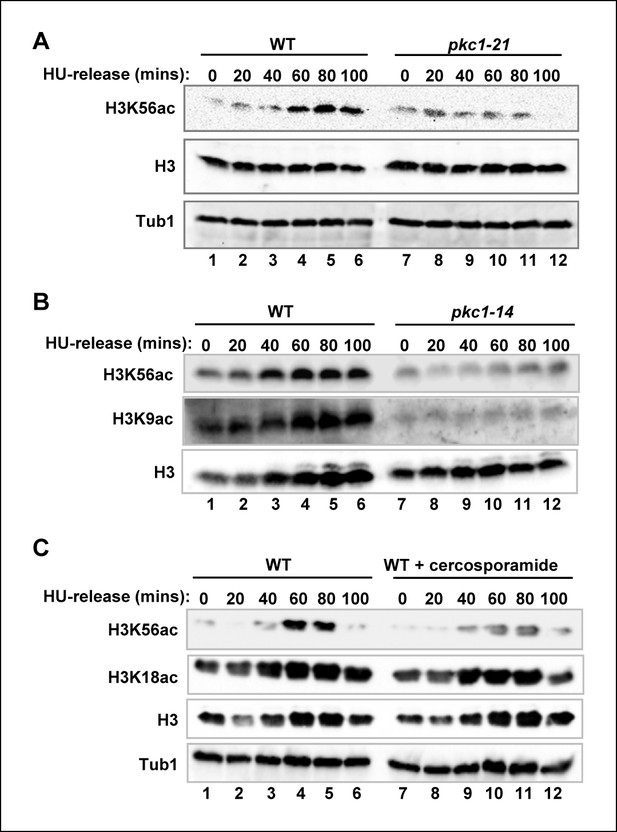
Pkc1p controls H3K56 acetylation.
(A-C) Western blot analysis of the H3K56 acetylation levels in (A) WT (DK186) or pkc1-21 (DK1697) or (B) WT (DK186) or pkc1-14 (DK1690) strains grown in YPD in the presence 1M sorbitol at 34ºC, treated with 200 mM HU for 2.5 hrs and released into YPD with sorbitol for the indicated times. (C) WT (DK186) cells grown in the presence or absence of cercosporamide at 30ºC in YPD, exposed to 200 mM HU for 2.5 hrs and released from a HU block for the indicated times. H3K9 acetylation levels are also shown in (B) and Histone H3 and tubulin (Tub1) are shown as loading controls.
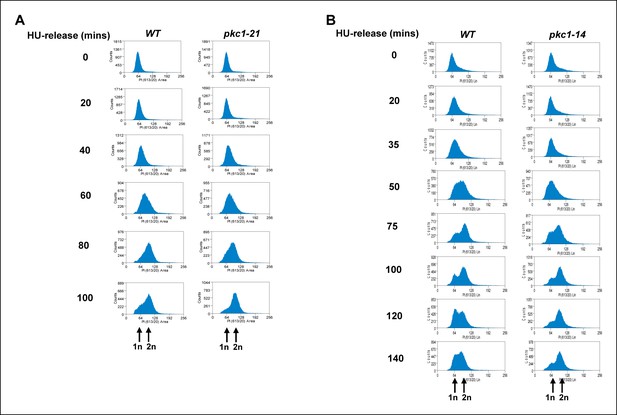
The pkc1-21 and pkc1-14 mutants exhibit a mitotic delay.
Cells from strains containing wild-type (WT) PKC1 (DK186) and the temperature sensitive pkc1-21 (DK1697) and pkc1-14 (DK1690) mutants were grown to early log phase at 30ºC in YPD supplemented with 1 M sorbitol, blocked with 200 mM HU for 2.5 hrs at 34ºC, washed and released into a fresh YPD media containing sorbitol at 34ºC. Aliquots were taken at the indicated times, analysed for propidium iodine staining by FACS, and the DNA content histograms were determined for at least 30,000 cells. The location of peaks corresponding to haploid (1n) and diploid (2n) DNA content are indicated.
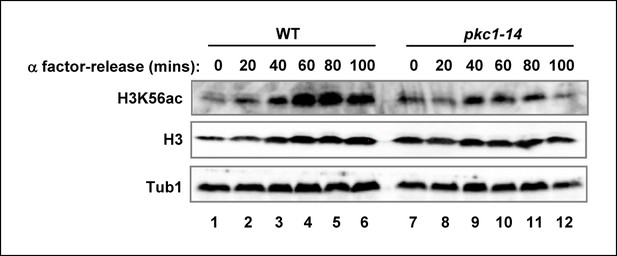
The role of Pkc1 in controlling H3K56 acetylation.
Western blot analysis of the H3K56 acetylation levels in WT (DK186) or pkc1-14 (DK1690) strains. Cells were released alpha factor block for the indicated times. Histone H3 and tubulin (Tub1) are shown as loading controls. All experiments were performed at 34ºC in YPD plus sorbitol.
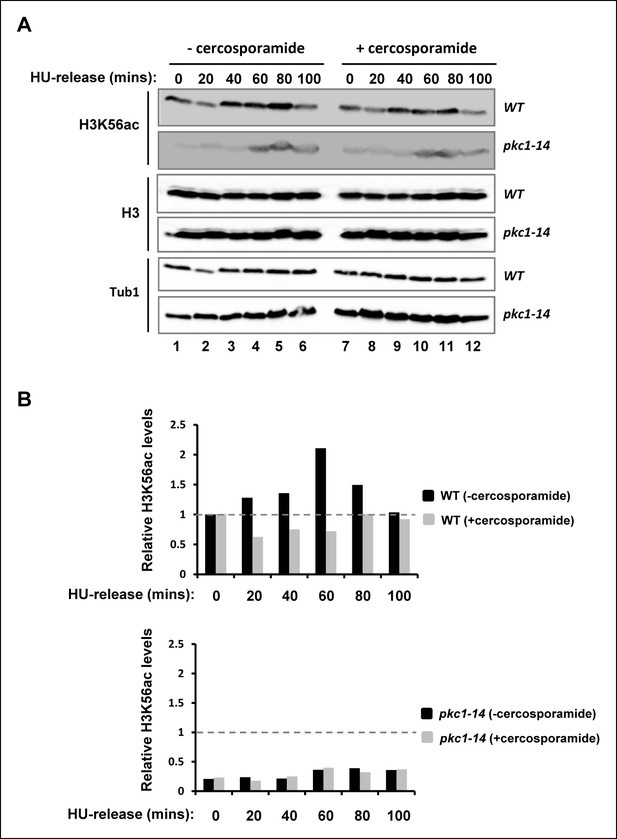
Comparison of genetic and pharmacological disruption of Pkc1 activity on H3K56 acetylation.
(A) Western blot analysis of the H3K56 acetylation levels in WT (DK186)(top) or pkc1-14 (DK1690)(bottom) strains. Cells were grown in the presence or absence of cercosporamide at 34ºC in YPD plus 1M sorbitol and were released from a 200 mM HU block for the indicated times. Histone H3 and tubulin (Tub1) are shown as loading controls. (B) Quantification of the H3K56ac levels in (A) normalised for tubulin and total H3 levels and shown relative to the WT cells prior to release from HU block (taken as “1”; indicated by dashed line).
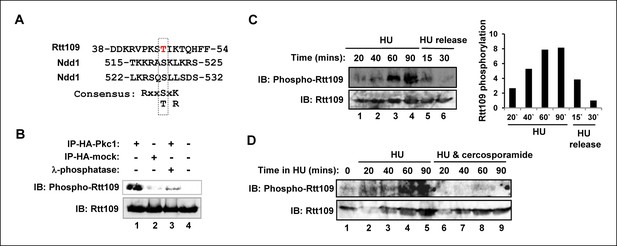
Pkc1-mediated Rtt109 phosphorylation.
(A) Location and local sequence contexts of the potential Pkc1p target site in Rtt109 (numbers indicate amino acid positions in the protein). (B) In vitro kinase assay using HA epitope-tagged Pkc1p immunoprecipitated from DZ2 cells and purified recombinant Rtt109-Vps75 protein complex. A HA IP from wild-type yeast cells was used as a control. Where indicated, λ phosphatase was added. Rtt109, HA-Pkc1 and phosphorylated Rtt109 T46 (phospho-Rtt109) were detected by immunoblotting (IB). (C and D) Rtt109 T46 phosphorylation in vivo. DZ5 cells were grown in SD media at 30ºC, treated with 200 mM HU for the indicated times and either (C) released from the HU block for the indicated times or (D) treated with or without cercosporamide. Rtt109 and phosphorylated Rtt109 T46 were detected by IB. Quantification of Rtt109 phosphorylation from (C) relative to total Rtt109 levels is shown on the right.
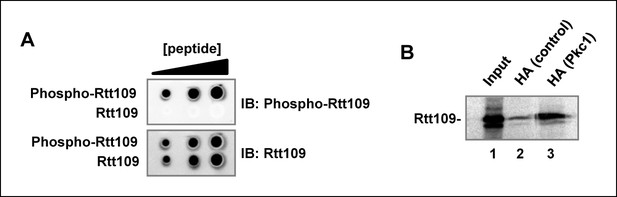
Pkc1 and Rtt109 phosphorylation.
(A) A phospho-specific antibody specifically recognises a phosphorylated peptide surrounding T46. A modified (phosphorylation at T46; phospho-Rtt109) DDKRVPKST(P)IKTC or non-modified peptide (Rtt109) DDKRVPKSTIKTC were dotted in increasing concentrations (2, 5, 10 µg) onto Hybond-C nitrocellulose membranes and immunoblotting was carried out with 1:1000 dilutions of antibodies to the phosphorylated or non-phosphorylated form of Rtt109. (B) Pkc1 interacts with Rtt109 in vitro. Immunoprecipitation assay of in vitro translated Rtt109 with anti-HA antibody bound to HA-tagged Pkc1 immunoprecipitated from DZ2 strain or with an anti-HA antibody mock immunoprecipitate from a wild-type strain lacking HA-tagged Pkc1 as a negative control.
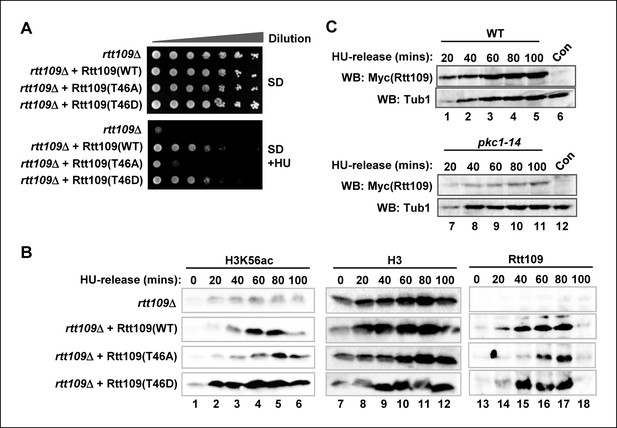
Rtt109 phosphorylation is needed for efficient H3K56 acetylation.
(A) Cell growth assays. 10 fold serial dilutions of rtt109Δ (Y01490) cells containing plasmids expressing WT or the indicated Rtt109 mutants, were plated onto SD media in the presence or absence of 100 mM HU. (B) Western blot analysis of the H3K56 acetylation (ac) levels in rtt109Δ cells containing empty vector (DZ8) or plasmids expressing WT or the indicated Rtt109 mutants (DZ5-7) grown in SD media at 30ºC and released from a 200 mM HU block for the indicated times. Total Rtt109 and Histone H3 levels are also shown. (C) Western blot analysis of Rtt109 levels in (WT) (DK186) or pkc1-14 (DK1690) cells containing empty vector (con) or a plasmid containing Myc-tagged Rtt109, grown in SD media in the presence of 1 M sorbitol at 34ºC and released from a 200 mM HU block for the indicated times.
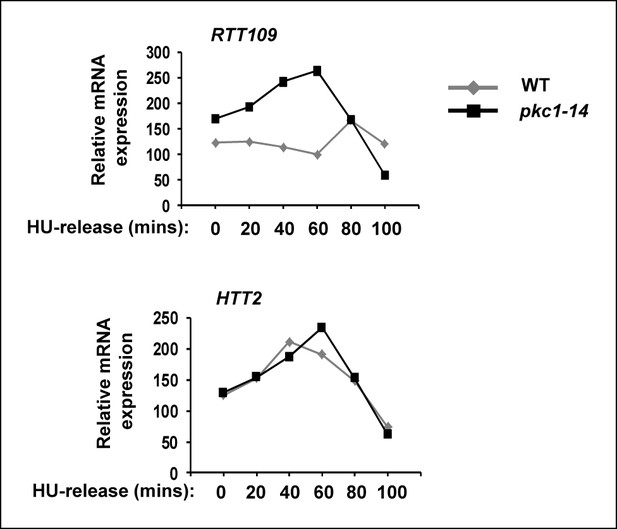
RTT109 expression is elevated in pkc1-14 cells.
RT-PCR analysis of RTT109 and HTT2 (control) expression in wild-type (WT)(DK186) (grey lines) or pkc1-14 (DK1690) (black lines) cells released from 200 mM HU block for the indicated times in YPD plus sorbitol at 34ºC. Data are shown relative to 18S rRNA levels.
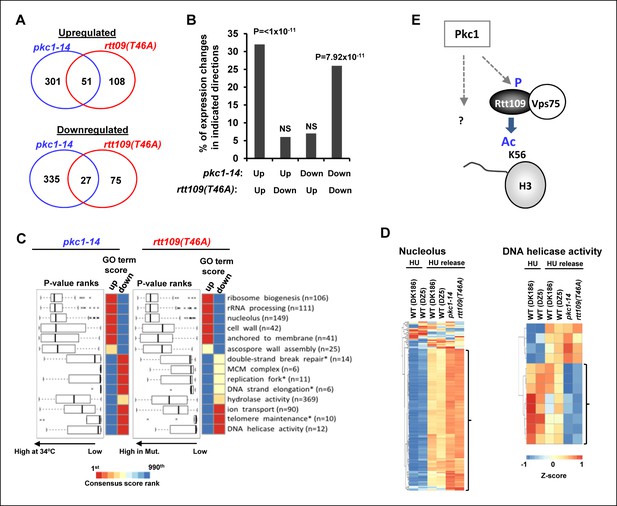
Pkc1 and RTT109 phosphorylation mutants generate overlapping gene expression defects.
(A) Venn diagrams showing the overlap of significantly upregulated (top) or downregulated (bottom) genes in pkc1-14 (DK1690) or rtt109(T46A) (DZ6) cells released from a 200 mM HU block. The experiment was performed by growing all strains in SD media supplemented with 1M sorbitol at 34ºC. (B) Prevalence of gene expression changes showing the indicated directionality of change (shown as a percentage of genes changed in the rtt109(T46A) mutant strain). Hypergeometric P-values are shown (NS = non-significant). (C) Boxplots (left) and heatmaps (right) showing the rank orders of significantly changing GO terms associated with genes whose expression is consistently changed in the pkc1-14 (DK1690) (left) or rtt109(T46A) (DZ6) (right) cells. The GO terms are ranked according to changes in the rtt109(T46A) (DZ6) mutant. In the heat maps, the columns indicate the P-value ranking of GO terms associated with genes that consistently increase (left) or decrease (right) their expression in the respective mutant strain. (D) Heat map showing the relative expression of the genes contained in the 'Nucleolus' and 'DNA helicase' categories across all conditions analysed. DK186 and DZ5 are the WT equivalent strains for the pkc1-14 (DK1690) and rtt109(T46A) (DZ6) mutant strains. Brackets indicate genes that are consistently up- or down-regulated in both mutant strains upon release from a HU block. (E) Model for Pkc1 function through phosphorylating Rtt109 and promoting its ability to acetylate (Ac) histone H3 K56. Question mark indicates additional activities of Pkc1.
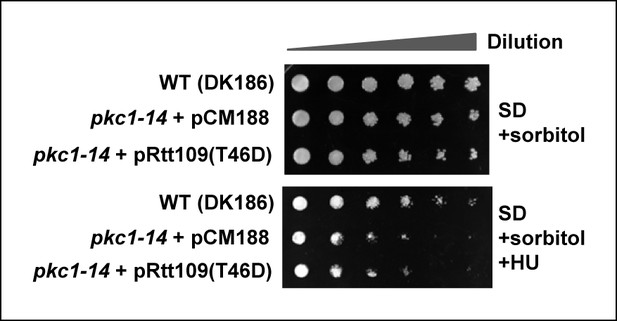
Rescue of the HU sensitivity of pkc1-14 by a rtt109 phosphomimetic allele.
10-fold serial dilutions of wild-type (WT; DK186), pkc1-14 (DK1690) containing empty vector pCM188 or pAS4106 (expressing Rtt109[T46D]) were spotted onto SD plates in the presence of sorbitol with or without 100 mM HU and incubated at 34°C for 3 days.
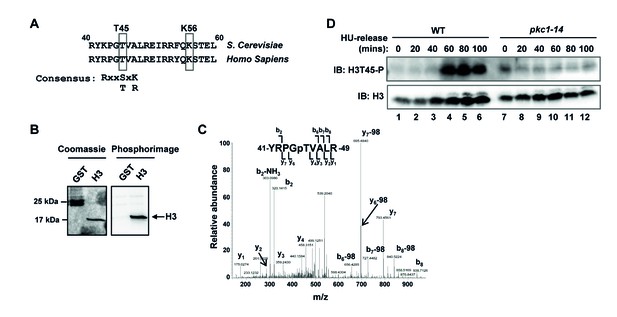
Pkc1 promotes histone H3 T45 phosphorylation.
(A) Location and local sequence contexts of the Pkc1p target site in histone H3 (numbers indicate amino acid positions in the protein and human H3 is shown below). (B) In vitro kinase assay using HA epitope-tagged Pkc1p immunoprecipitated from DZ2 cells and recombinant histone H3 or GST as substrates. Protein phosphorylation was visualised by phosphorimaging (right panel) and total input proteins by Coomassie blue staining (left panel). (C) Product ion spectra for the doubly-charged precursor ion [556.78]2+. The spectra is fully annotated, and includes both b- and y-ions that support phosphorylation (p) at the residue indicated, Thr45. (D) Histone H3 T45 phosphorylation in vivo. WT (DK186) or pkc1-14 (DK1690) cells were grown in SD media in the presence of 1M sorbitol at 34ºC and were treated with 200 mM HU and released from the HU block for the indicated times. Histone H3 and phosphorylated H3 T45 (H3T45-P) were detected by IB.
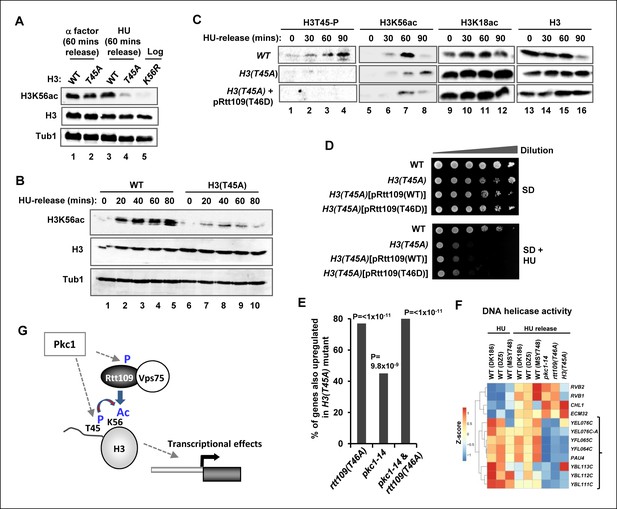
Phosphorylation of histone H3 T45 is required for efficient H3 K56 acetylation.
(A and B) Western blot analysis of K56 acetylation (H3K56Ac) and total H3 levels in (MSY748) cells containing plasmids expressing H3(WT) or the H3(T45A) mutant following release from an alpha factor or HU block for the indicated times. Cells were grown in SD media at 30ºC and treated with either alpha factor for 3 hrs or 200 mM HU for 2.5 hrs prior to release. Tubulin (Tub1) levels are shown as a loading control. Logarithmically growing (FXY19) cells containing a plasmid expressing the H3(K56R) mutant are shown as a control in (A). (C) Western blot analysis of T45 phosphorylation (H3T45-P), K56 acetylation (H3K56Ac), K18 (H3K18Ac) and total H3 levels in cells containing plasmids expressing H3(WT) (MSY748) or the H3(T45A) (H3-T45A) mutant following release from a HU block for the indicated times. Where indicated cells also expressed Rtt109(T46D). (D) Cell growth assays. 10 fold serial dilutions of cells containing plasmids expressing H3(WT) (MSY748) or the H3(T45A) (H3-T45A) plus either Rtt109(WT) or Rtt109(T46D), were plated onto SD media in the presence (bottom panel) or absence (top panel) of 100 mM HU. (E) Frequency of gene expression changes upregulated in the H3(T45A) mutant strain that are also upregulated in either the rtt109(T46A) or the pkc1-14 cells or in both of these mutant strains. Hypergeometric P-values are shown (NS = non-significant). (F) Heat map showing the relative expression of the genes contained in the 'DNA helicase' category across all conditions analysed. DK186, DZ5 and MSY748 are the WT equivalent strains for the pkc1-14 (DK1690), rtt109(T46A) (DZ6) and H3(T46A) mutant strains respectively. The experiment was performed by growing all strains in SD media supplemented with 1M sorbitol at 34ºC. Brackets indicate genes that are generally down-regulated in all of the mutant strains upon release from a HU block. (G) Model for Pkc1 function through phosphorylating Rtt109 and promoting its ability to acetylate (Ac) histone H3 K56 and acting through mediating H3 T45 phosphorylation which in turn enhances K56 acetylation.
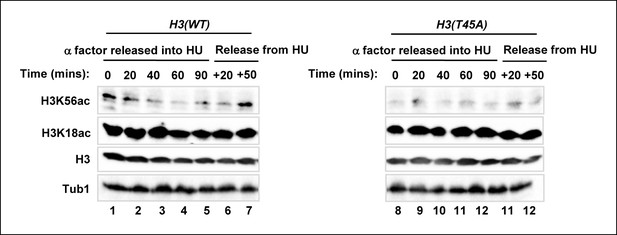
H3K56 acetylation is lost in the H3(T45A) mutant strain upon release from an alpha factor block.
Western blot analysis of H3K56 and H3K18 acetylation levels in MSY748 cells expressing wild-type H3(WT) or mutant H3(T45A). Cells were synchronised in G1 with a-factor and released into YPD media containing 200 mM HU for 90 min. Cells were then washed and released into fresh YPD media lacking HU for the indicated times and analysed by western blotting. Histone H3 and tubulin (Tub1) are shown as loading controls.
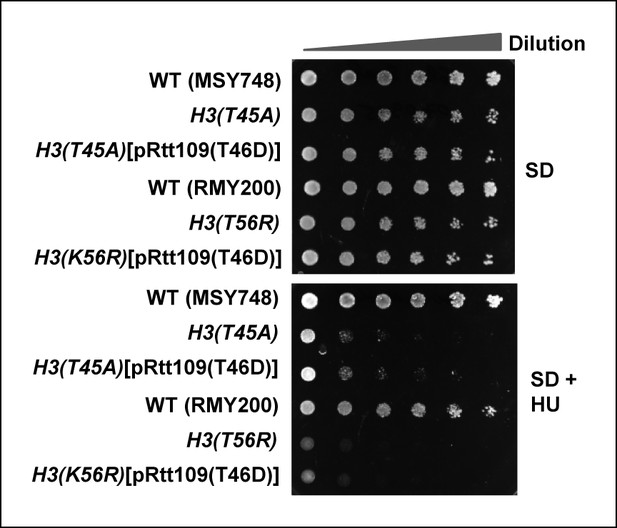
Cell growth assays of strains containing histone H3 mutants in the presence of phospho-mimetic versions of Rtt109.
10 fold serial dilutions of cells containing plasmids expressing H3(WT) (MSY748), H3(T45A) (H3-T45A) or H3(T45A) plus Rtt109(T46D) (top three rows), H3(WT) (RMY200), H3(K56R) (FXY19) or H3(K56R) plus Rtt109(T46D) (top three rows), were plated onto SD media containing 1 M sorbitol in the presence (bottom panel) or absence (top panel) of 100 mM HU.
Additional files
-
Supplementary fle 1
Yeast strains used in this study.
- https://doi.org/10.7554/eLife.09886.018





