Small molecule Photoregulin3 prevents retinal degeneration in the RhoP23H mouse model of retinitis pigmentosa
Figures
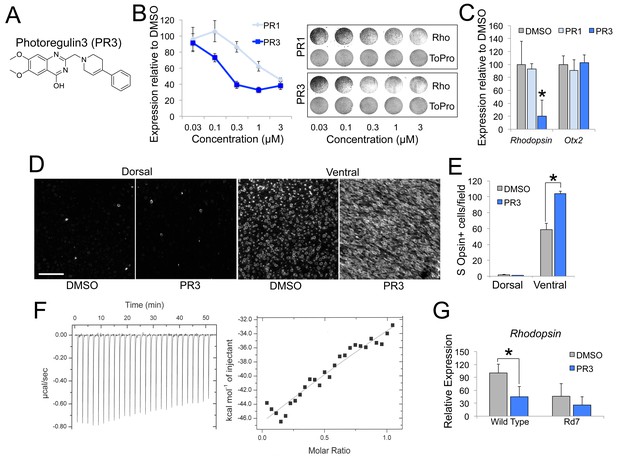
PR3 reduces rod gene expression via Nr2e3.
(A) Chemical structure of Photoregulin3 (PR3). (B) Dose-response relationship of PR1 and PR3 on Rhodopsin expression in dissociated retinal cell cultures (n = 3 for each concentration for each compound; value graphed is the mean ±SEM of 3 biological replicates). Example scans of Rhodopsin and ToPro3 staining from retinal cell cultures are shown on the right. (C) Confirmation of PR3’s potency by qPCR from intact retinal explant cultures from P4 mice treated with DMSO or 0.3 μM PR1 or PR3 for 2 days (n = 3–4 for each compound; value graphed is the mean ±SEM of the biological replicates, *p=0.0075 for DMSO vs. PR3, two-tailed t-test assuming equal variance using ΔCT values). (D) Intact retinas from P11 mice were explanted in media containing DMSO or 0.3 μM PR3 for 3 DIV and then stained for S Opsin in a whole-mount preparation. Scale bar represents 50 μm. (E) PR3-treated retinas had more S Opsin+ cells per 100 μm x 100 μm field in the ventral, but not dorsal, retina compared to DMSO-treated retinas (n = 3 biological replicates; value graphed is the mean ±SEM of the biological replicates, *p=0.00063, two-tailed t-test assuming equal variance). (F) Isothermal titration calorimetric study of PR3 binding to Nr2e3. (G) Intact retinas from adult (P23–P35) wild type and Rd7 were explanted in media containing DMSO or 1 μM PR3 for 2 days and Rhodopsin expression was measured by qPCR. PR3 decreased Rhodopsin in wild type retinas but not in Rd7 retinas (n = 4 biological replicates; value graphed is the mean ±SEM of the biological replicates, *p=0.033, two-tailed t-test assuming equal variance for wild type and p=0.13, two-tailed t-test assuming equal variance for Rd7 using ΔCT values).
-
Figure 1—source data 1
Source data for Figure 1.
- https://doi.org/10.7554/eLife.30577.006
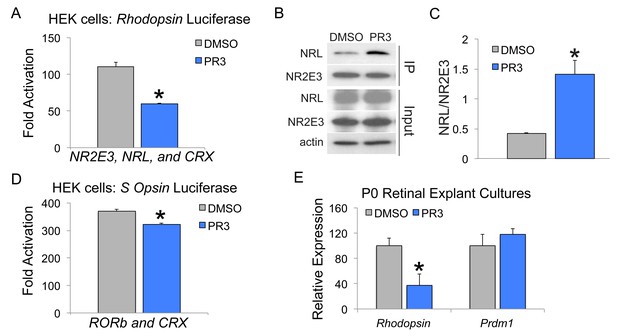
Effects of PR3 on photoreceptor promoter activation and co-activator binding.
(A) HEK 293 cells were transfected with NR2E3, CRX, and NRL and BR-225Luc (firefly luciferase driven by the bovine Rhodopsin promoter) and pRL-CMV (renilla luciferase driven by the CMV promoter; internal transfection control) and then treated with DMSO or 1 μM PR3 for 2 days. PR3 decreased Rhodopsin promoter activity after transfection of NR2E3, CRX, and NRL (n = 4 separate transfections per condition, values graphed are the mean ±SEM of the biological replicates, p<0.001, two-tailed t-test assuming equal variance). (B) HEK 293 cells were transfected with NR2E3 and NRL and then treated with DMSO or 1 μM PR3 for 2 days. Lysates were immunoprecipitated with an anti-Nr2e3 antibody and then analyzed by Western blot analysis. (C) PR3 treatment increased the binding between Nr2e3 and Nrl (n = 3 separate transfections per condition, values graphed are the mean ±SEM of the biological replicates, p=0.013, two-tailed t-test assuming equal variance). (D) HEK 293 cells were transfected with RORb, CRX, and S Opsin Luc (firefly luciferase driven by the S Opsin promoter) and pRL-CMV (renilla luciferase driven by the CMV promoter; internal transfection control) and then treated with DMSO or 1 μM PR3 for 2 days. PR3 decreased S Opsin promoter activity (n = 4 separate transfections per condition, values graphed are the mean ±SEM of the biological replicates, p=0.0014, two-tailed t-test assuming equal variance). (E) Retinas from P0 mice were explanted in media containing DMSO or 0.3 μM PR3 for 2 days. PR3 treatment reduced Rhodopsin expression (n = 4 biological replicates, values graphed are the mean ±SEM of the biological replicates, p=0.00081, two-tailed t-test assuming equal variance), but not Prdm1 expression, a direct target of RORb (n = 4 biological replicates, values graphed are the mean ±SEM of the biological replicates, p=0.41, two-tailed t-test assuming equal variance).
-
Figure 1—figure supplement 1—source data 1
Source data for Figure 1—figure supplement 1.
- https://doi.org/10.7554/eLife.30577.005
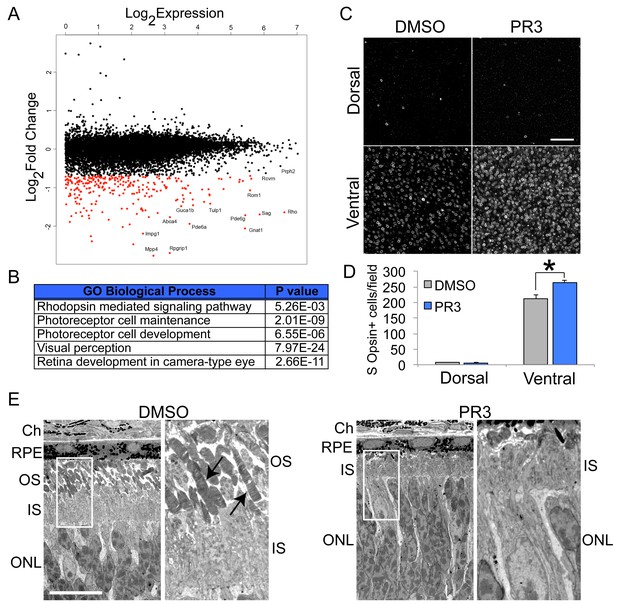
In vivo characterization of PR3 treatment.
(A) RNA sequencing results plotting log2FoldChange against log2Expression (RPKM) of wild type mice treated with DMSO vehicle or 10 mg/kg PR3 shows robust reduction in rod photoreceptor genes. (n = 2 mice per condition; values graphed are the mean of the two biological replicates) (B) Gene ontology analysis (http://geneontology.org/page/go-enrichment-analysis) results for largest changes (top 100) in gene expression assessed by RNA sequencing. (C) Whole mount S Opsin staining of retinas from mice treated with 10 mg/kg PR3 or DMSO vehicle for 3 days. Scale bar represents 25 μm. (D) Retinas from mice treated with PR3 had more S Opsin+ cells per 100 μm x 100 μm field in the ventral, but not dorsal, retina compared to controls (n = 4 biological replicates; value graphed is the mean ±SEM of the biological replicates, *p=0.0086, two-tailed t-test assuming equal variance). (E) Electron microscope micrographs of retinal sections of wild type mice treated with DMSO or 10 mg/kg PR3 (n = 2 mice per condition). Compared to DMSO controls, PR3 retinas have arrested outer segment development (arrows indicate outer segments in DMSO treated mice). Scale bare represents 10 μm.
-
Figure 2—source data 1
Source data for Figure 2.
- https://doi.org/10.7554/eLife.30577.008
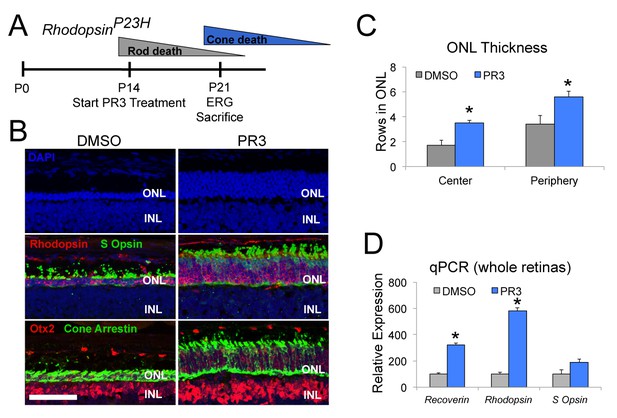
PR3 slows the progression of photoreceptor degeneration in the RhoP23H mouse model of RP.
(A) Timeline of photoreceptor degeneration in the RhodopsinP23H mouse and experimental design. (B) Immunofluorescence staining for Rhodopsin, S Opsin, Otx2, and Cone Arrestin on retinal sections from RhodopsinP23H mice demonstrate preservation of photoreceptors with PR3 treatment. Scale bar represents 50 μm. (C) Counts for rows of DAPI+ cells in the central and peripheral ONL show greater survival of photoreceptors with PR3 treatment (n = 7 mice for DMSO treatment and 8 mice for PR3; values graphed are the mean ±SEM of the biological replicates, p=0.0014 for center and 0.015 for periphery, two-tailed t-test assuming equal variance). (D) qPCR on whole retinas from RhodopsinP23H mice treated with DMSO or PR3 shows greater expression of photoreceptor genes Recoverin, Rhodopsin, and S Opsin with PR3 treatment (n = 6 mice for DMSO and 7 mice for PR3; values graphed are the mean ±SEM of the biological replicates, p=1.73E-05 for Recoverin, 3.13E-05 for Rhodopsin, and 0.076 for S Opsin, two-tailed t-test assuming equal variance on ΔCT values).
-
Figure 3—source data 1
Source data for Figure 3.
- https://doi.org/10.7554/eLife.30577.010
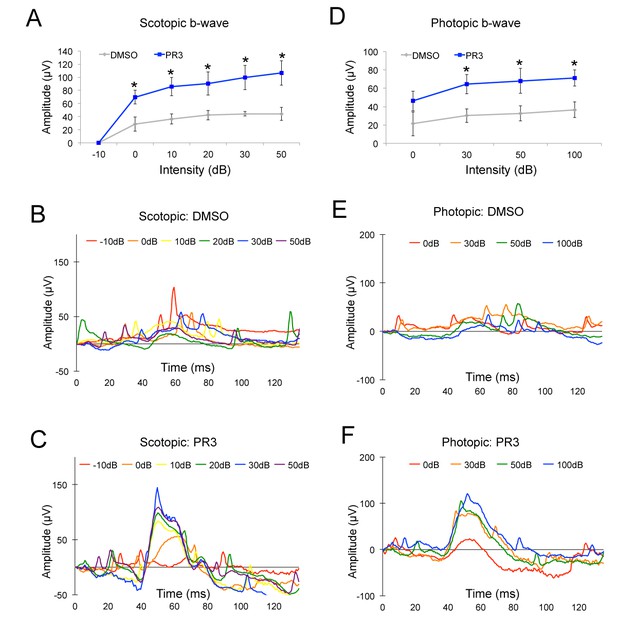
PR3 preserves visual function in the RhodopsinP23H mouse.
(A) Scotopic b-wave amplitudes from P21 RhodopsinP23H mice treated with 10 mg/kg PR3 or DMSO vehicle (n = 4 mice for DMSO treatment and 8 mice for PR3 treatment, values graphed are the mean ±SEM of the biological replicates, p=0.025, 0.012, 0.032, 0.021, and 0.015 for 0, 10, 20, 30, and 50 Decibels (dB) respectively, two-tailed t-test assuming unequal variance). (B) Representative scotopic ERG waveforms from a single DMSO vehicle mouse. (C) Representative scotopic ERG waveforms from a single PR3–treated mouse. (D) Photopic b-wave amplitudes from P21 RhodopsinP23H mice treated with 10 mg/kg PR3 or DMSO vehicle (n = 4 mice for DMSO treatment and 8 mice for PR3 treatment, values graphed are the mean ±SEM of the biological replicates, p=0.18, 0.025, 0.048, and 0.021 for 0, 30, 50, and 100 dB respectively, two-tailed t-test assuming unequal variance). (E) Representative photopic ERG waveforms from a single control mouse. (F) Representative photopic ERG waveforms from a single PR3-treated mouse.
-
Figure 4—source data 1
Source data for Figure 4.
- https://doi.org/10.7554/eLife.30577.012
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Transgenic mice (mus musculus), both sexes | Rhodopsin-Pro23His | The Jackson Laboratory | Jackson Stock No: 017628; RRID:IMSR_JAX:017628 | |
| Transgenic mice (mus musculus), both sexes | Nr2e3-Rd7 | The Jackson Laboratory | Jackson Stock No: 004643; RRID:IMSR_JAX:004643 | |
| C57Bl6J mice (mus musculus), both sexes | Wild type | The Jackson Laboratory | Jackson Stock No: 000664; RRID:IMSR_JAX:000664 | |
| Chemical compound | Photoregulin3 (PR3) | ChemDiv, and then synthesized in Ding Lab | PubChem CID: 2092851 | |
| Chemical compound | Photoregulin1 (PR1) | ChemDiv, and then synthesized in Ding Lab | PubChem CID: 7901316 | |
| Antibody | Anti- Rhodopsin (Rho4D2), mouse monoclonal | Dr. Robert Molday, UBC | RRID:AB_2315273 | 1:250 |
| Antibody | Anti-S Opsin, goat polyclonal | SCBT | Sc-14363; RRID:AB_2158332 | 1:400 |
| Antibody | Anti Cone Arrestin, rabbit polyclonal | Millipore | AB15282; RRID:AB_1163387 | 1:1000 |
| Antibody | Anti-Otx2, goat polyclonal | R&D Systems | AF1979; RRID:AB_2157172 | 1:200 |
| Antibody | Anti-Nr2e3, mouse monoclonal | R&D Sysems: | PP-H7223-00; RRID:AB_2155481 | 1:10,000 |
| Antibody | Anti-Nrl, rabbit polyclonal | Chemicon | Ab5693 | 1:10,000 |
| Antibody | Anti-beta actin, mouse monoclonal | Abcam | Ab6276; RRID:AB_2223210 | 1:20,000 |
| Recombinant DNA | HsCD00084154 (Nr2e3) | DNASU | HsCD00084154 | |
| Recombinant DNA | BR-225Luc | Dr. Shiming Chen, Washington University; PMID: 15689355 | ||
| Recombinant DNA | S Opsin 600 pGL3 | Dr. Douglas Forrest, NIH; PMID: 16574740 | ||
| Recombinant DNA | pRL-CMV | Promega | E6931 | |
| Recombinant DNA | hNRL-pCMVSport6 | Open Biosystems | MHS1010-58005 | |
| Recombinant DNA | hCRX-pCMVSport6 | Open Biosystems | MHS1010-73672 | |
| Recombinant DNA | hNR2E3-pcDNA3.1/HisC | Dr. Shiming Chen, Washington University; PMID: 15689355 | ||
| Recombinant DNA | HsCD00329674 (RORB) | DNASU | HsCD00329674 |
Additional files
-
Transparent reporting form
- https://doi.org/10.7554/eLife.30577.013





