Neurogliaform cortical interneurons derive from cells in the preoptic area
Abstract
Delineating the basic cellular components of cortical inhibitory circuits remains a fundamental issue in order to understand their specific contributions to microcircuit function. It is still unclear how current classifications of cortical interneuron subtypes relate to biological processes such as their developmental specification. Here we identified the developmental trajectory of neurogliaform cells (NGCs), the main effectors of a powerful inhibitory motif recruited by long-range connections. Using in vivo genetic lineage-tracing in mice, we report that NGCs originate from a specific pool of 5-HT3AR-expressing Hmx3+ cells located in the preoptic area (POA). Hmx3-derived 5-HT3AR+ cortical interneurons (INs) expressed the transcription factors PROX1, NR2F2, the marker reelin but not VIP and exhibited the molecular, morphological and electrophysiological profile of NGCs. Overall, these results indicate that NGCs are a distinct class of INs with a unique developmental trajectory and open the possibility to study their specific functional contribution to cortical inhibitory microcircuit motifs.
https://doi.org/10.7554/eLife.32017.001eLife digest
Our brain contains over a 100 billion nerve cells or neurons, and each of them is thought to connect to over 1,000 other neurons. Together, these cells form a complex network to convey information from our surroundings or transmit messages to designated destinations. This circuitry forms the basis of our unique cognitive abilities.
In the cerebral cortex – the largest region of the brain – two main types of neurons can be found: projection neurons, which transfer information to other regions in the brain, and interneurons, which connect locally to different neurons and harmonize this information by inhibiting specific messages. The over 20 different types of known interneurons come in different shapes and properties and are thought to play a key role in powerful computations such as learning and memory.
Since interneurons are hard to track, it is still unclear when and how they start to form and mature as the brain of an embryo develops. For example, one type of interneurons called the neurogliaform cells, have a very distinct shape and properties. But, until now, the origin of this cell type had been unknown.
To find out how neurogliaform cells develop, Niquille, Limoni, Markopoulos et al. used a specific gene called Hmx3 to track these cells over time. With this strategy, the shapes and properties of the cells could be analyzed. The results showed that neurogliaform cells originate from a region outside of the cerebral cortex called the preoptic area, and later travel over long distances to reach their final location. The cells reach the cortex a few days after their birth and take several weeks to mature.
These results suggest that the traits of a specific type of neuron is determined very early in life. By labeling this unique subset of interneurons, researchers will now be able to identify the specific molecular mechanisms that help the neurogliaform cells to develop. Furthermore, it will provide a new strategy to fully understand what role these cells play in processing information and guiding behavior.
https://doi.org/10.7554/eLife.32017.002Introduction
Cortical microcircuit function relies on the coordinated activity of a variety of GABAergic interneuron subtypes, which play critical roles in controlling the firing rate of glutamatergic pyramidal neurons, synchronizing network rhythms and regulating behavioral states (Cardin et al., 2009; Fu et al., 2014; Kepecs and Fishell, 2014; Pfeffer et al., 2013; Pi et al., 2013; Pinto and Dan, 2015; Sohal et al., 2009; Zhang et al., 2014). Different subtypes of cortical interneurons (INs) emerge during development and their specification arises through the complex interaction of cell-intrinsic mechanisms and cell-extrinsic cues (Bartolini et al., 2013; Fishell and Rudy, 2011; Huang, 2014; Kessaris et al., 2014). Cortical INs are generated in a variety of subpallial regions and the combinatorial expression of transcription factors (TFs) in these domains is believed to play a critical role in their fate specification (Kessaris et al., 2014; Anastasiades and Butt, 2011; Flames et al., 2007; Wonders and Anderson, 2006). The largest fraction (about 60–70%) of cortical INs is generated from NKX2.1-expressing progenitors located in the medial ganglionic eminence (MGE) (Butt et al., 2008; Xu et al., 2008) and their specification is under the control of the TFs LHX6 (Du et al., 2008; Liodis et al., 2007) and SOX6 (Azim et al., 2009; Batista-Brito et al., 2009). MGE-derived INs develop into fast-spiking parvalbumin (PV)+ basket and chandelier cells, as well as into Martinotti and multipolar somatostatin (SST)+ INs (Butt et al., 2008; Xu et al., 2008; Du et al., 2008; Butt et al., 2005; Fogarty et al., 2007; Taniguchi et al., 2013). The second largest fraction of cortical INs arises from the caudal ganglionic eminence (CGE) (Miyoshi et al., 2010; Nery et al., 2002) and expresses TFs such as PROX1, SP8 and NR2F2 (Cai et al., 2013; Ma et al., 2012; Miyoshi et al., 2015; Rubin and Kessaris, 2013). CGE-derived INs also express the ionotropic serotonin receptor 3A (5-HT3AR) and give rise to a large diversity of INs, including reelin+ cells, vasointestinal peptide (VIP)+/calretinin+ bipolar cells and VIP+/cholecystokinin+ basket cells (Miyoshi et al., 2010; Armstrong and Soltesz, 2012; Prönneke et al., 2015; Lee et al., 2010; Murthy et al., 2014; Vucurovic et al., 2010). Finally, lineage-tracing experiments using Hmx3 (Nkx5.1)-Cre (Gelman et al., 2009) and Dbx1-Cre driver lines (Gelman et al., 2011) have shown that a small fraction (about 10%) of cortical INs originate from the preoptic area (POA) (Gelman et al., 2009; Gelman et al., 2011).
Among cortical INs, neurogliaform cells (NGCs) display unique characteristics. They represent the main source of ‘slow’ cortical inhibition by acting on metabotropic GABAB receptors (Tamás et al., 2003), and are thought to be key effectors of a powerful inhibitory circuit recruited by long-range connections such as interhemispheric and thalamic projections (Craig and McBain, 2014; Palmer et al., 2012; De Marco García et al., 2015). Whether the current description of NGCs captures an IN subtype related to a distinct developmental specification process is unclear. Here we used in vivo genetic lineage-tracing to follow the developmental origin and trajectory of NGCs. We found that they originate from a distinct pool of 5-HT3AR-expressing Hmx3+ cells located in the rostral POA region, ventrally to the anterior commissure. In the embryonic POA, Htr3a-GFP+ INs in the Hmx3+ domain expressed CGE-enriched TFs such as PROX1 and NR2F2, but only rarely, if not, MGE-related TFs such as NKX2.1 or LHX6. In the cortex, Hmx3-derived Htr3a-GFP+ INs expressed markers of CGE-derived INs such as NPY and/or reelin, as well as CGE-enriched TFs such as SP8, NR2F2 and PROX1, but neither MGE-specific markers such as PV or SST nor TFs such as LHX6 or SOX6. Finally, single-molecule in situ hybridization and electrophysiological recordings followed by post hoc reconstructions indicated that Hmx3-derived Htr3a-GFP+ cells exhibited the molecular, electrophysiological and morphological profile of NGCs. Taken together, these results demonstrate that cortical NGCs have a precise developmental trajectory that is linked to the expression of the transcription factor (TF) Hmx3 in a discrete embryonic subpallial region.
Results
To determine whether the POA could contribute to Htr3a-GFP INs, we crossed Htr3a-GFP; R26R-tdTOMfl/fl mice with Hmx3-Cre mice, a reporter line previously shown to fate map a population of cortical INs derived from cells located in the POA (Gelman et al., 2009). Examination of brains from Hmx3-Cre::Htr3a-GFP; R26R-tdTOMfl/fl matings at embryonic age 14.5 (E14.5) revealed a large fraction of Hmx3; tdTOM+ cells co-labelled with Htr3a-GFP (85.2 ± 0.9%; 1675/1986 cells in the overlap zone) in a restricted region of the POA, located ventrally to the anterior commissure (Figure 1A,C, Figure 1—source data 1). Individual co-labelled Hmx3; tdTOM+/Htr3a-GFP+ cells displaying migratory profiles were observed at more caudal levels entering the CGE (Figure 1B), suggesting that a fraction of Hmx3; tdTOM+/Htr3a-GFP+ cells migrate from the POA into the CGE. In situ hybridization indicated that the vast majority (95.8 ± 0.9%; 115/120 cells) of Hmx3; tdTOM+/Htr3a-GFP+ cells located in the POA expressed the endogenous Htr3a mRNA, in contrast to Hmx3; tdTOM+cells negative for Htr3a-GFP (0.5 ± 0.5%; 1/158 cells) (Figure 1D, Figure 1—source data 1). In addition, a large fraction of Hmx3; tdTOM+/Htr3a-GFP+ cells in the POA expressed the TFs PROX1 (54.0 ± 2.2%; 249/463 cells) and NR2F2 (65.9 ± 1.3%; 795/1212 cells), which have previously been shown to be enriched in CGE-derived INs (Cai et al., 2013; Ma et al., 2012; Miyoshi et al., 2015; Rubin and Kessaris, 2013), but more rarely the TF NKX2.1 (15.3 ± 1%; 175/1146 cells) (Figure 1E,F, Figure 1—source data 1). Collectively, these results indicate that a fraction of Hmx3+ cells located in the POA express the 5-HT3AR and a pattern of TFs related to CGE-derived INs.
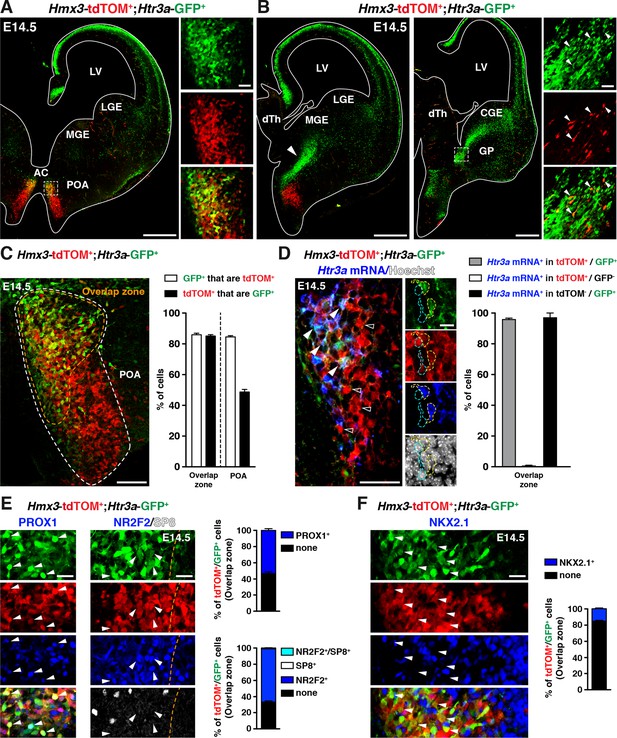
A fraction of 5-HT3AR-expressing interneurons (INs) originates from Hmx3-derived cells in the preoptic area (POA) and expresses transcription factors related to the caudal ganglionic eminence (CGE).
(A) At E14.5, tdTOM specifically labels cells expressing Hmx3 (Hmx3; tdTOM+). Htr3a-GFP+ INs co-label with tdTOM in a rostral region of the POA (dashed lines; high magnified images) located ventrally to the anterior commissure (AC). (B) At more caudal levels, Hmx3; tdTOM+/Htr3a-GFP+ embryonic cells appear to further migrate caudally (arrowhead) towards the CGE. High magnified images show Hmx3; tdTOM+/Htr3a-GFP+ cells entering the ventral CGE (dashed lines). (C) More than 80% of Hmx3; tdTOM+ cells co-label with Htr3a-GFP (and conversely) in the overlap zone (orange dashed line) of the POA domain defined by Hmx3; tdTOM recombination (white dashed line). (D) In situ hybridization showing that almost all Hmx3; tdTOM+/Htr3a-GFP+ INs in the POA express the Htr3a mRNA (arrowheads; yellow outline), whereas Hmx3; tdTOM+ cells do not (empty arrowheads; cyan outline). (E) In the overlap zone of the POA, IHC reveals that Hmx3; tdTOM+/Htr3a-GFP+ embryonic cells express (arrowheads) the CGE-enriched transcription factors PROX1 (left) and NR2F2 but not SP8 (right). (F) By contrast, the vast majority of Hmx3; tdTOM+/Htr3a-GFP+ INs do not express NKX2.1 (arrowheads). dTh: dorsal thalamus, GP: globus pallidus, LGE: lateral ganglionic eminence, LV: lateral ventricle, MGE: medial ganglionic eminence. Scale bars: 300 µm in A, B: low magnified images; 100 µm in C, D: low magnified images; 50 µm in A, B: high magnified images; 25 µm in E, F; 10 µm in D: high magnified images.
-
Figure 1—source data 1
Detailed counts of cells quantified in Figure 1 in the different experimental conditions.
- https://doi.org/10.7554/eLife.32017.004
To determine whether Hmx3; tdTOM+/Htr3a-GFP+ cells in the POA eventually give rise to a specific subpopulation of cortical INs, we examined brains at various postnatal ages. From P5 to P21, Hmx3; tdTOM+/Htr3a-GFP+ INs were found distributed preferentially in superficial cortical layers and in a variety of other brain regions including in the hippocampus (Figure 2A, Figure 2—figure supplement 1). Hmx3; tdTOM+/Htr3a-GFP+ cells were rarely observed at postnatal ages in the subpallial brain regions corresponding to the embryonic POA (i.e., the preoptic nuclei) (Figure 2—figure supplement 2). In situ hybridization for Htr3a mRNA indicated that Hmx3; tdTOM+/Htr3a-GFP+ cells expressed the Htr3a transcript, similarly to Htr3a-GFP+ cells negative for Hmx3; tdTOM (Figure 2C). About half (51.9 ± 2.1%; 863/1653 cells) of Hmx3-derived cells in the cortex were co-labelled with Htr3a-GFP+ and virtually all Hmx3; tdTOM+/Htr3a-GFP+ (96.1 ± 0.5%; 357/372 cells) were positive for the neuronal marker NeuN (Figure 2D,E, Figure 2—source data 1). In contrast, the fraction of Hmx3; tdTOM+ cells negative for Htr3a-GFP mostly did not express NeuN (3.4 ± 1.3%; 28/758 cells) (Figure 2D,E, Figure 2—source data 1), and remained relatively constant across postnatal ages (Figure 2—figure supplement 3A, Figure 2—figure Supplement 3—source data 1). These cells displayed the morphology of glial cells and expressed the astrocytic markers GFAP and S100β as well as the oligodendrocytic marker SOX10 (Figure 2—figure supplement 3B–D). Overall, these findings indicate that the cortical Hmx3-derived lineage observed in the POA differentiate into INs that are Htr3a-GFP+, glial cells that are Htr3a-GFP negative and, for a small fraction, to NeuN+ neurons negative for Htr3a-GFP.
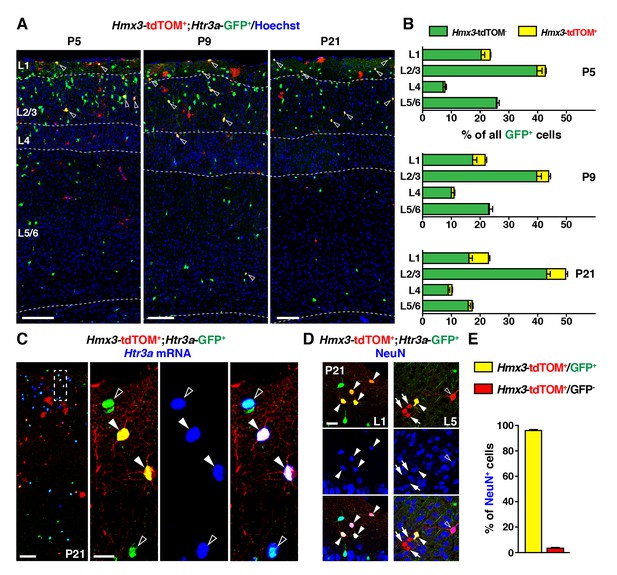
During the postnatal period, Hmx3-derived cells from the preoptic area (Gelman et al., 2009) constitute a small but persistent fraction of 5-HT3AR-expressing interneurons (INs) (Lee et al., 2010) in superficial cortical layers.
(A–B) Hmx3; tdTOM+/Htr3a-GFP+ cells represent a fraction of Htr3a-GFP+ INs that increases along postnatal ages P5 (left), P9 (middle) and P21 (right). Note that double-labeled cells (open arrowheads) are mainly located in superficial cortical layers. (C) In situ hybridization showing that, at P21, Htr3a mRNA is found in cortical Hmx3; tdTOM+/Htr3a-GFP+ INs (arrowheads) as in Htr3a-GFP+ INs (open arrowheads). (D–E) Hmx3; tdTOM+/Htr3a-GFP+ cells largely express the neuronal marker NeuN (arrowheads) whereas those negative for Htr3a-GFP only rarely do (arrows, open arrowhead). Scale bars: 100 µm in A, C: low magnified images; 25 µm in C: high magnified images, D.
-
Figure 2—source data 1
Detailed counts of cells quantified in Figure 2 in the different experimental conditions.
- https://doi.org/10.7554/eLife.32017.010
A second distinct region in the POA expressing Dbx1 was previously reported to give rise to subsets of cortical INs (Gelman et al., 2011). To determine whether a fraction of Htr3a-GFP+ INs also originate from Dbx1-expressing cells, we examined Dbx1-Cre::Htr3a-GFP; R26R-tdTOMfl/fl brains at postnatal periods. While the overall contribution of Hmx3-derived cells to the Htr3a-GFP IN population in the cortex increased with postnatal maturation from P5 (6.8 ± 0.2%; 77/1118 cells) to P21 (16.0 ± 0.3%; 863/5405 cells) (Figure 3A,C), only minimal fractions (1.44 ± 0.2%; 81/5741 cells at P5; 0.8 ± 0.2%; 20/2551 cells at P21) of Htr3a-GFP+ INs were fate-mapped with Dbx1; tdTOM (Figure 3B,C, Figure 3—source data 1). Moreover, Dbx1; tdTOM+ cells were preferentially found in deep cortical layers and expressed the MGE-enriched TF SOX6 (30.4 ± 2.2%; 82/266 cells), while PROX1 was found only in a very small fraction of Dbx1; tdTOM+ cells expressing also the Htr3a-GFP (2.2 ± 0.7%; 6/266 cells) (Figure 3D, Figure 3—source data 1). In addition, Dbx1; tdTOM+ INs expressed Lhx6 mRNA (Figure 3E), and the MGE-related markers SST and PV (Figure 3—figure supplement 1), and only very rarely the Htr3a mRNA (Figure 3F). Overall, these results indicate that Hmx3-derived 5-HT3AR+ cortical INs largely originate from Hmx3-expressing cells but not from the Dbx1+ domain, which gives rise to INs expressing MGE-related markers.
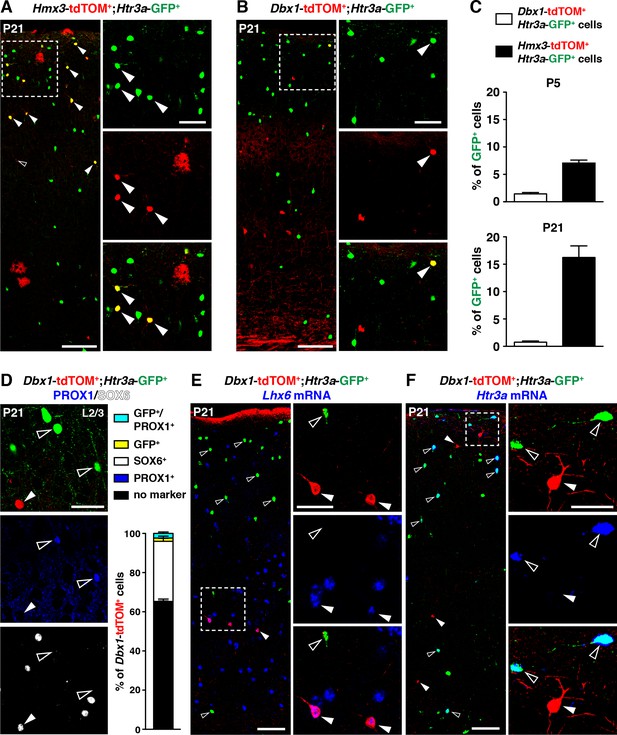
5-HT3AR-expressing interneurons (INs) largely originate from Hmx3+ but not Dbx1+ cells.
(A–C) A consistent fraction of cortical Htr3a-GFP+ INs co-labels with Hmx3; tdTOM (A, arrowheads) at P5 and P21, whereas only a minimal fraction does with Dbx1; tdTOM (B, arrowhead). (D) Dbx1; tdTOM+ INs express the MGE-enriched TF SOX6 (arrowhead) but not the CGE-enriched TF PROX1 (open arrowheads). Only a minimal fraction of Dbx1; tdTOM+ co-labelled for Htr3a-GFP, among which the majority were PROX1+. (E–F) In situ hybridization showing that Dbx1; tdTOM+ INs express the transcript for the MGE-enriched TF Lhx6 (E, arrowheads) whereas Htr3a-GFP+ INs do not (E, open arrowheads). In contrast, Dbx1; tdTOM+ INs do not express the Htr3a transcript (F, arrowheads) whereas Htr3a-GFP+ INs do (F, open arrowheads). Scale bars: 100 µm in A, B, E, F: low magnified images; 50 µm in A, B, D, E, F: high magnified images.
-
Figure 3—source data 1
Detailed counts of cells quantified in Figure 3 in the different experimental conditions.
- https://doi.org/10.7554/eLife.32017.013
We next examined whether Hmx3; tdTOM+/Htr3a-GFP+ cells expressed distinct patterns of TFs involved in cortical IN subtype specification. At P21, we found that, similarly to Htr3a-GFP+ INs, a large fraction (65.8 ± 3.4%; 202/308 cells) of Hmx3; tdTOM+/Htr3a-GFP+ INs expressed the CGE-enriched but not the MGE-related TFs. Indeed, a large fraction of them (65.8 ± 3.4%; 202/308 cells) expressed PROX1 but not SOX6 (Figure 4A,B, Figure 4—source data 1), as well as NR2F2 (32.7 ± 5.9%; 71/218 cells), SP8 (9.8 ± 2.6%; 22/218 cells), and both SP8 and NR2F2 (8.8 ± 2.0%; 18/218 cells) (Figure 4C,D, Figure 4—source data 1). The fraction of Hmx3; tdTOM+/Htr3a-GFP+ expressing at least one of these two latter TFs was smaller and biased toward NR2F2 expression, when compared to Htr3a-GFP+ INs (Figure 4D, Figure 4—figure supplement 1, Figure 4—source data 1). These findings indicate that Hmx3; tdTOM+/Htr3a-GFP+ cortical INs express a repertoire of TFs related to CGE but not to MGE-derived INs.
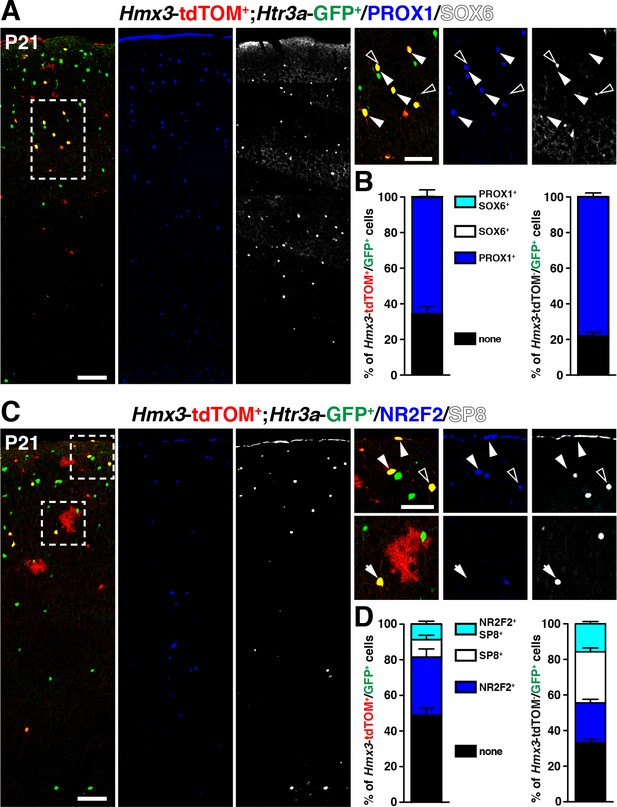
Hmx3; tdTOM+/Htr3a-GFP+ cortical interneurons (INs) express markers related to the CGE but not to the MGE.
(A–B) Hmx3; tdTOM+/Htr3a-GFP+ INs express the CGE-enriched TF PROX1 (A; arrowheads) but not the MGE-related TF SOX6 (A; open arrowheads) similarly to Htr3a-GFP+ INs that do not derive from Hmx3+ cells (B, right graph). (C–D) Hmx3; tdTOM+/Htr3a-GFP+ cells express the CGE-enriched TFs NR2F2 (arrowheads) and (open arrowheads)/or SP8 (arrow). Htr3a-GFP+ derived from Hmx3+ cells are biased towards NR2F2 expression, in comparison to Htr3a-GFP+ INs that do not co-label with Hmx3; tdTOM (D, blue bars). Scale bars: 100 µm in A, C: low magnified images; 50 µm in A, C: high magnified images.
-
Figure 4—source data 1
Detailed counts of cells quantified in Figure 4 in the different experimental conditions.
- https://doi.org/10.7554/eLife.32017.016
We next examined whether Hmx3; tdTOM+/Htr3a-GFP+ INs expressed classical CGE markers such as reelin, NPY and VIP (Lee et al., 2010; Murthy et al., 2014; Vucurovic et al., 2010). Quantification across layers revealed that a large fraction of Hmx3; tdTOM+/Htr3a-GFP+ INs expressed reelin or NPY. This was particularly striking in layer 1 (L1) for reelin (Figure 5A,C) and in L2–6 for NPY (Figure 5B,E), respectively (Figure 5—source data 1). Overall, Hmx3; tdTOM+/Htr3a-GFP+ INs accounted for approximately a third of all reelin+/Htr3a-GFP+ INs (34.5 ± 2.3%; 267/797 cells) and of all NPY+/Htr3a-GFP+ INs (27.7 ± 2.3%; 149/571 cells) (Figure 5D,F, Figure 5—source data 1). Given that INs expressing reelin have been shown to co-express NPY (Lee et al., 2010), we assessed reelin and NPY co-expression in Hmx3; tdTOM+/Htr3a-GFP+ cells. At P21, only a small fraction (8.0 ± 0.9%; 17/232 cells) of these cells expressed NPY without reelin, thus indicating that reelin labels the largest fraction (66.1 ± 8.6%; 267/398 cells) of Hmx3-derived Htr3a-GFP+ INs (Figure 5G,H, Figure 5—source data 1). In contrast, Hmx3; tdTOM+/Htr3a-GFP+ INs did not co-label nor with the CGE-specific marker VIP (Figure 5I) neither with the MGE-enriched markers SST and PV (Figure 5—figure supplement 1). These results indicate that Hmx3-derived Htr3a-GFP+ INs mainly belong to the reelin but not to the VIP subtypes and account for an important fraction of all reelin+/Htr3a-GFP+ INs.
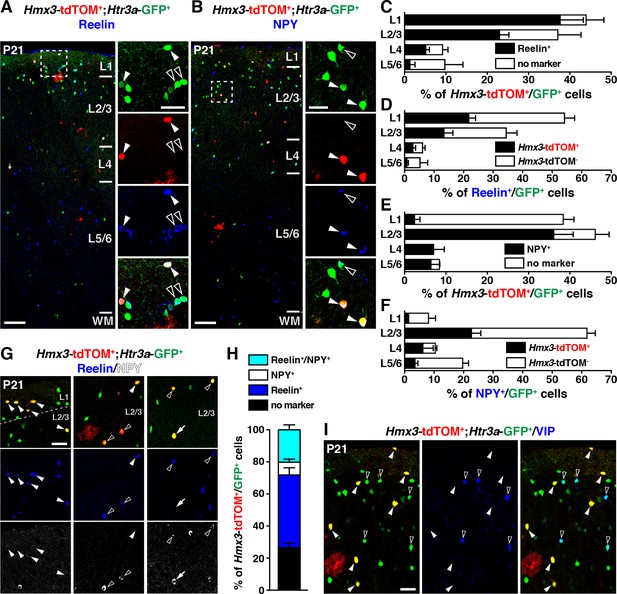
Hmx3; tdTOM+/Htr3a-GFP+ cortical interneurons (INs) express reelin and NPY but not VIP.
(A–F) Hmx3; tdTOM+/Htr3a-GFP+ INs are stained with the neurochemical markers reelin (A, arrowheads) and NPY (B, arrowheads) as well as Htr3a-GFP+ cells negative for Hmx3; tdTOM (open arrowheads). Note that reelin-positive Hmx3; tdTOM+/Htr3a-GFP+ INs are preferentially found in L1-3 (C) whereas NPY-expressing Hmx3; tdTOM+/Htr3a-GFP+ INs are mainly found in L2-6 (E). Hmx3; tdTOM+/Htr3a-GFP+ INs account for more than one third of all reelin-positive Htr3a-GFP+ (D) and of all NPY-positive Htr3a-GFP+ cells (F). (G–H) Hmx3; tdTOM+/Htr3a-GFP+ INs mainly express reelin (G, arrowheads) or reelin and NPY (G, open arrowheads) but to a smaller extend only NPY (G, arrow). (I) Hmx3; tdTOM+/Htr3a-GFP+ INs do not express VIP (arrowheads), whereas Htr3a-GFP+ INs negative for Hmx3; tdTOM do (open arrowheads). WM: white matter. Scale bars: 100 µm in A, B: low magnified images; 50 µm in A, B: high magnified images, G, I.
-
Figure 5—source data 1
Detailed counts of cells quantified in Figure 5 in the different experimental conditions.
- https://doi.org/10.7554/eLife.32017.019
Two distinct profiles of reelin-expressing INs have been identified in L1 of the neocortex, namely neurogliaform (NGCs) and single bouquet-like cells (SBCs) (Cadwell et al., 2016; Jiang et al., 2015). At a molecular level, NGCs are strongly enriched in the carbonic anhydrase 4 (Car4) transcript in contrast to SBCs (Cadwell et al., 2016). Using single-molecule fluorescent in situ hybridization experiments (Wang et al., 2012), we found that Hmx3; tdTOM+/Htr3a-GFP+ INs in L1 exhibited significantly higher levels of Car4 transcripts in contrast to Htr3a-GFP+ INs (Figure 6A, Figure 6—source data 1), thus indicating that Hmx3-derived Htr3a-GFP+ INs share the molecular profile of NGC. To further verify whether their morphological and electrophysiological features could fit with NGCs, we performed whole-cell recordings (Figure 6B) and reconstructions (Figure 6C,D; Figure 6—figure supplement 1) of Hmx3-derived versus non Hmx3-derived Htr3a-GFP+ INs in L1 of the barrel cortex. There, Hmx3; tdTOM+/Htr3a-GFP+ INs displayed the characteristic morphology of elongated NGCs with dense axonal ramifications mostly restricted to L1 (Figure 6C, Figure 6—figure supplement 1), whereas Htr3a-GFP+ INs negative for tdTOM had the morphology of SBCs with less developed axonal processes that extended deeper in cortical layers (Figure 6D). With regard to their first action potential (AP) at rheobase, NGCs are reported to display a type 1 profile with only an after-hyperpolarization potential (AHP), whereas SBCs show a type 2 profile consisting of an AHP followed by an after-depolarization potential (ADP) (Cadwell et al., 2016; Jiang et al., 2015). Strikingly, all (29 out of 29) recorded Hmx3; tdTOM+/Htr3a-GFP+ INs were of type 1, thus confirming their NGC identity. Moreover, the vast majority of them (23 out of 29) were of type 1A with a deep and wide AHP and only a few (6 out of 29) were of type 1B with a shallow and narrow AHP (Figure 6E, left, Figure 6—source data 2). Htr3a-GFP+ INs negative for Hmx3; tdTOM had more variable profiles, but the majority of them (24 out of 28) were displaying a type two profile with an average ADP amplitude of 2.30 ± 0.51 mV, suggesting that they were SBCs. Most of them (20 out of 28) were of type 2B with a small ADP below the spike threshold, a few others (4 out of 28) were of type 2A with a big ADP above the spike threshold (Figure 6E, right, Figure 6—source data 2) and another few of them (4 out of 28) had not measurable ADP (not shown). Hmx3; tdTOM+/Htr3a-GFP+ INs showed also a higher tendency to late-spiking when compared to Htr3a-GFP+ INs (Figure 6F, Figure 6—source data 2). Hmx3; tdTOM+/Htr3a-GFP+ INs had bigger AP delay average, but not significantly different from Htr3a-GFP+ INs negative for Hmx3; tdTOM (Figure 6—figure supplement 2C, Figure 6—source data 2). However, the variability of individual cell values was higher for Hmx3; tdTOM+/Htr3a-GFP+ INs, indicating that these cells tend to be more late-spiking, a characteristic of NGCs (Cadwell et al., 2016; Jiang et al., 2015). Alignement of the first APs at rheobase revealed other putative differences between the two groups (Figure 6G, Figure 6—source data 2). After quantification, Hmx3; tdTOM+/Htr3a-GFP+ INs significantly differed from Htr3a-GFP+ INs negative for Hmx3; tdTOM in the first AP amplitude (Peak), AHP amplitude (AHP), membrane resistance (Rm) (Figure 6H, Figure 6—source data 2) and threshold potential (Vthr) (Figure 6—figure supplement 2C). We next aimed to determine whether Hmx3 and non Hmx3-derived Htr3a-GFP+ INs classes in L1 could be predicted from single-cell electrophysiological properties. Using an automatic cell type classifier based on combined electrophysiological measures, we were able to predict the Hmx3-derived class with 80.7% accuracy, with highest weights found on ADP, Peak and AHP but not Vthr (Figure 6I,J; Figure 6—figure supplement 2A,B, Figure 6—source data 2). Finally, we analysed Hmx3; tdTOM+/Htr3a-GFP+ INs in other cortical layers to determine whether they displayed the same NGC characteristics. Similarly to L1 cells, Car4 expression in L2-6 was significantly higher in Hmx3; tdTOM+/Htr3a-GFP+ INs as compared to Htr3a-GFP+ INs negative for tdTOM (Figure 6—figure supplement 3A,B, Figure 6—source data 1). Morphological recovery of Hmx3; tdTOM+/Htr3a-GFP+ INs located in L2–6 revealed that all cells (7 out of 7) had also the characteristic morphology of NGCs (Figure 6—figure supplement 4). Furthermore, characteristic properties of NGC like the tendency to late spiking, the depth of AHP, and the level of Vthr were significantly more pronounced in these cells compared to L1 cells (Figure 6—figure supplement 3C,E). Overall, these data indicate that Htr3a-GFP+ INs displaying the molecular, morphological and electrophysiological properties of NGC INs originate from Hmx3-expressing cells in the embryonic POA (Figure 7, orange), whereas SBCs in layer 1, as well as VIP +INs, are more likely to originate from the CGE (Figure 7, green).
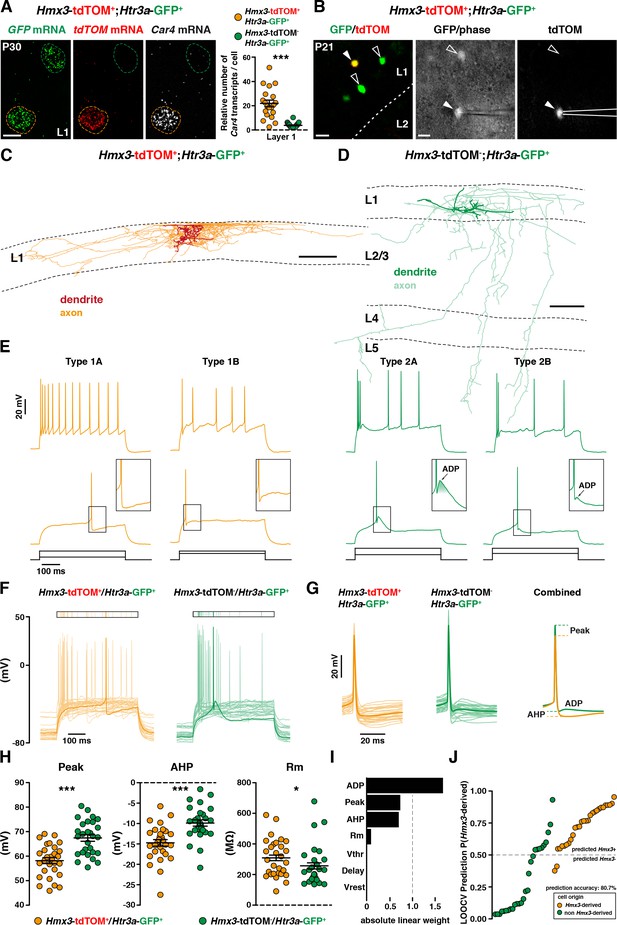
Hmx3; tdTOM+/Htr3a-GFP+ cortical interneurons (INs) in layer 1(L1) display the molecular, morphological and electrophysiological features of neurogliaform cells (NGCs).
(A) RNAscope multiplex fluorescent hybridization for tdTOM, GFP and Car4 transcripts on P30 brains showing that L1 Hmx3; tdTOM+/Htr3a-GFP+ INs express Car4 at significantly higher levels (orange outline) as compared to Htr3a-GFP+ INs negative for tdTOM (green outline) (***p<0.0001; Mann-Whitney test). (B) Example of a L1 Hmx3; tdTOM+/Htr3a-GFP+ cell (arrowhead) or Htr3a-GFP+ INs negative for tdTOM (open arrowheads) checked before patching (left image). Example of a patched Hmx3; tdTOM+/Htr3a-GFP+ IN (middle and right images, arrowhead). (C) Illustrative reconstruction of a Hmx3; tdTOM+/Htr3a-GFP+ IN in L1 displaying the characteristic morphology of an elongated NGC with dense axonal ramifications restricted to L1. (D) Illustrative reconstruction of a Htr3a-GFP+ IN negative for tdTOM in L1 displaying the characteristic morphology of single bouquet-like cell (SBC) with axonal ramifications extending deep into L5. (E) Illustrative traces from recorded Hmx3; tdTOM+/Htr3a-GFP+ INs (orange) and Htr3a-GFP+ INs negative for tdTOM (green), showing the first action potentials (APs) at rheobase and trains of APs at higher current injections. (F) Superimposed single AP traces at rheobase of all Hmx3; tdTOM+/Htr3a-GFP+ INs (orange) and Htr3a-GFP+ INs negative for tdTOM (green). Thick traces correspond to type 1A and type 2A examples in E. (G) Same traces as in (F), aligned to the AP, with a lower time scale. Thin lines are individual cell traces and thick lines are trace averages. The average traces on the right are aligned to the threshold potential (Vthr). (H) Plots of AP peak amplitude (Peak; ***p<0.0001; unpaired t-test), after hyperpolarization potential amplitude (AHP; ***p<0.0001; unpaired t-test) and membrane resistance (Rm; *p=0.0318; Mann-Whitney test) showing significant differences between the two cell types. (I) Absolute linear weights assigned by the classification model trained on all cells with standardized electrophysiological properties. (J) Prediction probabilities estimated by the classifier on the cell left out in the leave-one-out-cross-validation (LOOCV) loop. Cells are ordered on the x-axis by origin and prediction value, and the color code reflect their origin. Cells above the probability threshold 0.5 are more likely to be Hmx3-derived according to the model. Scale bars: 10 µm A; 20 µm in B; 100 µm C, D.
-
Figure 6—source data 1
Detailed counts of cells expressing Car4 quantified in Figure 6 in the different experimental conditions.
- https://doi.org/10.7554/eLife.32017.025
-
Figure 6—source data 2
Electrophysiological properties of cells quantified in Figure 6.
- https://doi.org/10.7554/eLife.32017.026
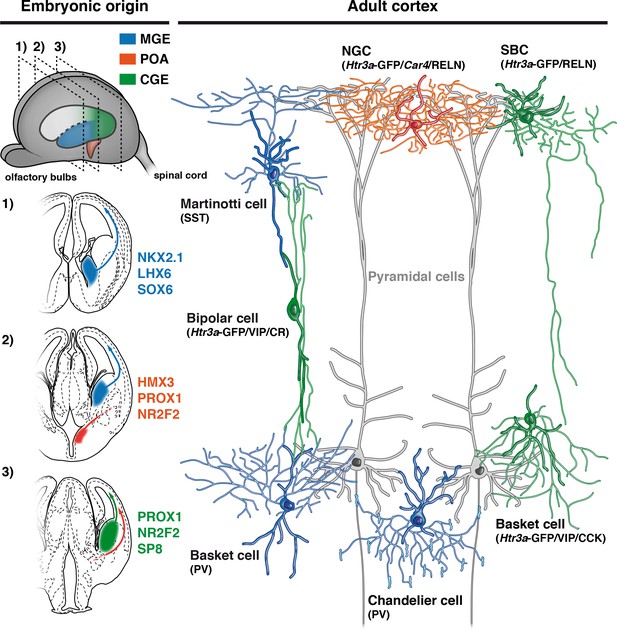
Developmental origin of cardinal classes of cortical interneurons.
The Martinotti somatostatin (SST) cell, the parvalbumin (PV) basket cell and the PV+ chandelier cell originate from NKX2.1+ progenitors of the medial ganglionic eminence (MGE, blue) and rely on the transcription factors (TFs) LHX6 and SOX6. The Htr3a-GFP/reelin (RELN) single-bouquet cell (SBC), the Htr3a-GFP/vasointestinal peptide (VIP)/cholecystokinin (CCK) basket cell and the Htr3a-GFP/VIP/calretinin (CR) bipolar cell are derived from cells located in the caudal ganglionic eminence (CGE, green) that express the TFs PROX1, NR2F2 and SP8. The RELN/Car4/Htr3a-GFP neurogliaform cell (NGC) is specifically derived from Hmx3+ cells located in the preoptic area (POA, orange) and express the TFs PROX1 and NR2F2.
Discussion
Distinct subtypes of local GABAergic INs are required to regulate microcircuit function (Cardin et al., 2009; Fu et al., 2014; Kepecs and Fishell, 2014; Pfeffer et al., 2013; Pi et al., 2013; Pinto and Dan, 2015; Sohal et al., 2009; Zhang et al., 2014). Whether current classifications of cortical IN subtypes relate to intrinsic biological processes such as their developmental specification is a key question in the field (Huang, 2014; Taniguchi et al., 2013). Among cortical INs, NGCs are considered as belonging to a distinct subtype acting as a main effector of a powerful inhibitory motif recruited by long-range connections (Tamás et al., 2003; Craig and McBain, 2014; Palmer et al., 2012). Here we aimed to track the developmental trajectory of NGCs using genetic fate mapping strategies. We find that NGCs derive from Htr3a-GFP+/Hmx3+ cells located in the embryonic POA, but not from Dbx1+ cells. Strikingly, L1 Hmx3-derived Htr3a-GFP+ INs display the distinct molecular, morphological and electrophysiological properties of NGCs, whereas Htr3a-GFP+ INs negative for Hmx3 have the profile of SBCs (Cadwell et al., 2016). Hmx3-derived Htr3a-GFP+ NGCs represent about a third of reelin-expressing Htr3a-GFP+ INs. At a molecular level, Hmx3; tdTOM+/Htr3a-GFP+ NGCs express CGE-related TFs such as NR2F2 and PROX1, indicating that they share common features with CGE-derived INs. Overall, these results indicate that cortical NGCs derive from a discrete embryonic area located in the subpallial POA and that their specification is linked to the expression of the TF Hmx3.
Htr3a-GFP+ INs derived from Hmx3+ cells express CGE-enriched transcription factors
Here, we find that a fraction (about 15%) of Htr3a-GFP+ cortical INs originate from Hmx3+ but not Dbx1+ cells in the POA. The overall fraction of Hmx3; tdTOM+/Htr3a-GFP+ INs to the total Htr3a-GFP+ IN population in the cortex almost doubled from P9 to P21, a period during which neural migration is largely achieved. Given that about 40% of developing cortical INs undergo apoptosis during early postnatal life (Southwell et al., 2012) higher levels of programmed cell death in Htr3a-GFP+ INs negative for tdTOM could thus account for the relative postnatal increase in the cortical Hmx3; tdTOM+/Htr3a-GFP+ cell population. Overall, our data support the general view that the differential expression of TFs in progenitor cells originating from distinct subpallial germinal zones controls the specification of cortical IN subtypes (Huang, 2014; Kessaris et al., 2014; Anastasiades and Butt, 2011; Flames et al., 2007; Gelman et al., 2009). A striking example in the field relates to chandelier INs, which have been shown to derive from Nkx2.1+ cells produced specifically at late embryonic time-points in a restricted region of the MGE (Taniguchi et al., 2013). Three major germinal zones contribute to the generation of cortical INs, including the MGE, the CGE and the POA (Kessaris et al., 2014). The majority of cortical IN subtypes (about 60–70%) originates from Nkx2.1+ progenitors in the MGE and includes fast-spiking PV+ basket INs, chandelier cells and SST+ Martinotti cells. In addition to NKX2.1, sequential expression of the TFs such as LHX6 (Anastasiades and Butt, 2011; Du et al., 2008; Liodis et al., 2007) and SOX6 (Azim et al., 2009; Batista-Brito et al., 2009) controls the specification and migration of MGE-derived IN subtypes. Here we find that Htr3a-GFP+ cortical INs originating from Hmx3+ cells in the POA do not express MGE-enriched TFs such as LHX6 or SOX6. In the embryonic POA, we observe that only a small fraction of Hmx3; tdTOM+/Htr3a-GFP+ cells expresses the TF NKX2.1, which has been shown to be strongly expressed in the ventricular zone of the POA (Flames et al., 2007). This could be due to either down-regulation of NKX2.1 in postmitotic Hmx3; tdTOM+/Htr3a-GFP+ cells as previously observed in migrating MGE-derived INs (Nóbrega-Pereira et al., 2008) or to the fact that the majority of Hmx3; tdTOM+/Htr3a-GFP+ cells do not originate from NKX2.1 progenitors. In line with this second possibility, recent genetic fate-mapping experiments using a Nkx2.1-ires-Flpo knock-in mouse line did not appear to label INs in L1 (He et al., 2016). Overall, further work needs to be done to clarify the precise origin of mitotic cells giving rise to the pool of Hmx3; tdTOM+/Htr3a-GFP+ cells observed in the embryonic POA. In contrast to the absence of co-localization with MGE-enriched TFs, we find that Hmx3; tdTOM+/Htr3a-GFP+ INs express TFs such as PROX1 and NR2F2 in the embryonic POA and in the postnatal cortex. PROX1 and NR2F2 have been shown to be expressed in CGE cells and these TFs are maintained in subsets of cortical INs as they mature in the developing cortex (Cai et al., 2013; Ma et al., 2012; Rubin and Kessaris, 2013; Murthy et al., 2014; Kanatani et al., 2008). Our results thus indicate that the specification of Hmx3-derived and CGE-derived Htr3a-GFP+ INs shares common transcriptional controls and that the expression of Hmx3 in a fraction of Htr3a-GFP+ defines the distinct subtype of NGCs. To gain insights on the requirement of Hmx3 in the specification of Hmx3-derived Htr3a-GFP+ NGCs, cell-type specific genetic deletion strategies are needed. Finally, the molecular pathways specifically controlled by Hmx3 in NGCs remain to be identified.
Htr3a-GFP+ INs derived from Hmx3+ cells express CGE but not MGE-enriched neurochemical markers
MGE-derived INs express the neurochemical markers PV or SST and are preferentially distributed in lower cortical layers, whereas CGE-derived INs specifically express the 5-HT3AR, but not PV or SST, and populate more superficial cortical layers (Fishell and Rudy, 2011; Huang, 2014; Rudy et al., 2011). Using in situ hybridization, we confirmed that Hmx3+ lineage give rise to superficial cortical Htr3a-GFP+ INs expressing the Htr3a transcript. Reelin, VIP and NPY have been used as neurochemical markers to identify different subtypes of Htr3a-GFP+ cortical INs (Lee et al., 2010; Murthy et al., 2014; Vucurovic et al., 2010). Expressions of reelin and VIP are mutually exclusive in Htr3a-GFP+ INs, whereas a fraction of them is found to co-express reelin and NPY (Lee et al., 2010). Using these markers, we find that Hmx3; tdTOM+/Htr3a-GFP+ INs express reelin and/or NPY, but not VIP, PV or SST. This is in line with previous results showing that Hmx3+ INs express NPY and not VIP, PV or SST (Gelman et al., 2009). Finally, we find that cortical INs from the Dbx1+ domain express the MGE-enriched markers PV or SST and only rarely co-label with Htr3a-GFP+ INs. In addition, Dbx1-derived cortical INs express the MGE-related TFs SOX6 and LHX6 but not the CGE-enriched TF PROX1. Taken together, our findings thus indicate that Hmx3+ but not Dbx1+ cells give rise to a subpopulation of cortical Htr3a-GFP+ INs, which share molecular similarities with CGE but not MGE-derived INs. However, given that both Hmx3+ and Hmx3- Htr3a-GFP+ INs express reelin and/or NPY, these classical neurochemical markers are not sufficient to segregate Hmx3- and non-Hmx3- derived Htr3a-GFP+ IN subtypes.
Hmx3-derived Htr3a-GFP+ INs display the molecular, morphological and electrophysiological properties of NGCs
Electrophysiological recordings obtained from Htr3a-GFP+ INs revealed the existence of many different subtypes of INs (Lee et al., 2010). Recently, electrophysiological and morphological characterization of L1 INs combined to single-cell transcriptomics delineated two main types of INs, namely NGCs and SBCs (Cadwell et al., 2016). Our findings support this observation and indicate that Hmx3-derived Htr3a-GFP+ INs exhibit the morphological and electrophysiological signature of NGCs and strongly express Car4, a transcript present at high level in NGCs, but not in SBCs. In contrast, Htr3a-GFP+ INs in L1 that do not derive from Hmx3+ cells, have low levels of Car4 and display the electrophysiological profile of SBCs. These results indicate that Htr3a-GFP+ cortical INs in L1 can be subdivided in two major groups characterized by distinct intrinsic properties and that these subgroups are determined by their sites of origin and the differential expression of the TF Hmx3. Finally, we show that all Hmx3; tdTOM+/Htr3a-GFP+ INs analysed in deeper cortical layers also display molecular, morphological and electrophysiological profiles of NGCs, indicating that the Hmx3-Cre line labels NGCs across neocortical layers. In vivo studies of the canonical cortical microcircuit have mainly relied on the use of the mutually exclusive SST-, PV- and VIP-Cre driver lines (Cardin et al., 2009; Fu et al., 2014; Kepecs and Fishell, 2014; Pfeffer et al., 2013; Pi et al., 2013; Pinto and Dan, 2015; Sohal et al., 2009; Zhang et al., 2014) but they do not give access to NGCs. These cells are the main source of ‘slow’ GABAB-receptor mediated inhibition in the neocortex (Tamás et al., 2003) and are thought to constitute the core cellular component of a canonical inhibitory circuit in L1 (Craig and McBain, 2014). NGCs acts through GABAB-receptors to inhibit the activity of projection neurons and halt ongoing network activity through dendritic calcium channels (Craig and McBain, 2014). Long-range interhemispheric inhibition has been shown to be mediated through a GABAB-receptor dependent mechanism and it has been proposed that this process requires the recruitment of L1 cortical INs, possibly of the neurogliaform-type (Craig and McBain, 2014; Palmer et al., 2012). However, given the diversity of L1 cortical INs (Cadwell et al., 2016; Jiang et al., 2013) and the lack of molecular tools to specifically target NGCs in vivo, it has so far not been possible to manipulate and interrogate exclusively NGCs in cortical networks. Our findings redefine the Hmx3-Cre mice as a valuable tool to specifically investigate the functional contribution of NGCs in the cortical microcircuit motif.
Materials and methods
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Strain, strain background (Mus Musculus) | Tg(Htr3a-EGFP)DH30Gsat (referred as Htr3a-GFP) | GENSAT Consortium | MGI:3846657 | Maintained on a C57Bl/6 background |
| Strain, strain background (Mus Musculus) | B6.Cg-Gt(ROSA)26Sortm14(CAG-tdTomato)Hze/J (referred as R26R-tdTOMfl/fl) | The Jackson Laboratory | MGI:104735 | Maintained on a C57Bl/6 background |
| Strain, strain background (Mus Musculus) | Tg(Hmx3-icre)1Kess | provided by Oscar Marin | MGI:5566775 | Maintained on a C57Bl/6 background |
| Strain, strain background (Mus Musculus) | Dbx1tm2(cre)Apie (referred as Dbx1-Cre) | provided by Alessandra Pierani | MGI:3757955 | Maintained on a C57Bl/6 background |
| Antibody | Anti-GFAP, rabbit polyclonal | Abcam, United Kingdom | ab7260 | (1:2000) |
| Antibody | Anti-GFP, rabbit polyclonal | Millipore, Germany | AB3080 | (1:500) |
| Antibody | Anti-GFP, goat polyclonal | Abcam | ab5450 | (1:2000) |
| Antibody | Anti-NeuN (clone A60), mouse monoclonal | Millipore | MAB377 | (1:500) |
| Antibody | Anti-NKX2.1 (H-190), rabbit polyclonal | Santa Cruz Biotechnology, Dallas, TX | sc-13040 | (1:100) |
| Antibody | Anti-NPY, rabbit polyconal | Abcam | ab10980 | (1:500) |
| Antibody | Anti-NR2F2, rabbit polyclonal | Abcam | ab42672 | (1:500) antigen retrieval (Citrate buffer pH 6.0; 85°C; 20 min) |
| Antibody | Anti-PROX1, goat polyclonal | R&D System,Minneapolis, MN | AF2727 | (1:250) |
| Antibody | Anti-PV, mouse monoclonal | Swant, Switzerland | PV235 | (1:2000) |
| Antibody | Anti-Reelin, mouse monoclonal | Abcam | ab78540 | (1:500) |
| Antibody | Anti-S100β, rabbit polyclonal | Abcam | ab41548 | (1:2000) |
| Antibody | Anti-SST, rat monoclonal | Millipore | MAB354 | (1:500) |
| Antibody | Anti-SOX6, rabbit polyclonal | Abcam | ab30455 | (1:500) |
| Antibody | Anti-SOX10 (N-20), goat polyclonal | Santa Cruz Biotechnology | sc-17343 | (1:100) |
| Antibody | Anti-SP8 (C-18), goat polyclonal | Santa Cruz Biotechnology | sc-104661 | (1:50) antigen retrieval (Citrate buffer pH 6.0; 85°C; 20 min) |
| Antibody | Anti-tdTOM, goat polyclonal | Sicgen, Portugal | AB8181-200 | (1:500) |
| Antibody | Anti-VIP, rabbit polyclonal | Abcam | ab22736 | (1:500) ASCF perfusion; 2 hr PFA 4% postfixation |
| Antibody | Donkey anti-rabbit Alexa Fluor488 | Invitrogen, Carlsbad, CA | A21206 | (1:500) |
| Antibody | Donkey anti-goat Alexa Fluor488 | Invitrogen | A11055 | (1:500) |
| Antibody | Donkey anti-goat Alexa Fluor405 | Abcam | ab175664 | (1:500) |
| Antibody | Donkey anti-rabbit Alexa Fluor405 | Abcam | ab175651 | (1:500) |
| Antibody | Donkey anti-rabbit Alexa Fluor647 | Invitrogen | A31573 | (1:500) |
| Antibody | Donkey anti-rat Alexa Fluor647 | Invitrogen | A21247 | (1:500) |
| Antibody | Donkey anti-mouse Alexa Fluor647 | Invitrogen | A31571 | (1:500) |
| Antibody | Donkey anti-goat Alexa Fluor647 | Invitrogen | A21447 | (1:500) |
| Antibody | Streptavidin, Alexa Fluor647-conjugated | ThermoFisher/Invitrogen | S21374 | (1:500) |
| Antibody | Anti-Digoxigenin-AP, Fab fragment | Roche, Switzerland | 11082736103 | (1:2000) |
| Recombinant DNA reagent | Htr3a plasmid probe | Gift from Dr. B. Emerit | NA | Linearization: HindIII-HF; antisense synthesis: T7; concentration 1 µg |
| Recombinant DNA reagent | Lhx6 plasmid probe | Gift from Dr. M. Denaxa | NA | Linearization: Not1; antisense synthesis: T3; concentration 1 µg |
| Recombinant DNA reagent | RNAScope Probe-tdTomato-C2 | Affimetrix, Santa Clara, CA | 317041-C2 | |
| Recombinant DNA reagent | RNAScope Probe-EGFP | Affimetrix | 400281 | |
| Recombinant DNA reagent | RNAScope Probe-Car4-C3 | Affimetrix | 468421-C3 | |
| Sequence-based reagent | Genotyping PCR primer forB6.Cg-Gt(ROSA)26Sortm14(CAG-tdTomato)Hze/J | Microsynth, Switzerland (desalted;100 µM stock) | WT-F (oIMR9020): AAG GGA GCT GCA GTG GAG TA | https://www2.jax.org/protocolsdb/f?p=116:5:0::NO:5:P5_MASTER_PROTOCOL_ID,P5_JRS_CODE:29436,007909 |
| Sequence-based reagent | Genotyping PCR primer forB6.Cg-Gt(ROSA)26Sortm14(CAG-tdTomato)Hze/J | Microsynth (desalted;100 µM stock) | WT-R (oIMR9021): CCG AAA ATC TGT GGG AAG TC | |
| Sequence-based reagent | Genotyping PCR primer forB6.Cg-Gt(ROSA)26Sortm14(CAG-tdTomato)Hze/J | Microsynth (desalted;100 µM stock) | Mut-R (oIMR9103): GGC ATT AAA GCA GCG TAT CC | |
| Sequence-based reagent | Genotyping PCR primer forB6.Cg-Gt(ROSA)26Sortm14(CAG-tdTomato)Hze/J | Microsynth (desalted;100 µM stock) | Mut-F (oIMR9105): CTG TTC CTG TAC GGC ATG G | |
| Sequence-based reagent | Genotyping PCR primer for Tg(Htr3a-EGFP)DH30Gsat | Microsynth (desalted;100 µM stock) | Com-F (273): GCA AGA TGT GAC CAA GCC ACC TAT TT | http://www.med.unc.edu/mmrrc/resources/genotyping-protocols/mmrrc-273 |
| Sequence-based reagent | Genotyping PCR primer for Tg(Htr3a-EGFP)DH30Gsat | Microsynth (desalted; 100 µM stock) | WT-R: CAG CCC TCA GCC CTT TGA GAC TTA AG | |
| Sequence-based reagent | Genotyping PCR primer for Tg(Htr3a-EGFP)DH30Gsat | Microsynth (desalted; 100 µM stock) | Mut-R: TGA ACT TGT GGC CGT TTA CGT CG | |
| Sequence-based reagent | Genotyping PCR primer forTg(Hmx3-icre)1Kess | Microsynth (desalted;100 µM stock) | Mut-F: CTC TGA CAG ATG CCA GGA CA | |
| Sequence-based reagent | Genotyping PCR primer forTg(Hmx3-icre)1Kess | Microsynth (desalted;100 µM stock) | Mut-R: TCT CTG CCC AGA GTC ATC CT | |
| Sequence-based reagent | Genotyping PCR primer forDbx1tm2(cre)Apie | Microsynth (desalted;100 µM stock) | WT-F (1307): GCA AGG AAA TGT CTC TGG GAC | https://www.infrafrontier.eu/sites/infrafrontier.eu/files/upload/public/pdf/genotype_protocols/EM01924_geno.pdf |
| Sequence-based reagent | Genotyping PCR primer forDbx1tm2(cre)Apie | Microsynth (desalted;100 µM stock) | WT-R (1115): GAG GAT GAG GAA ATC ACG GTG | |
| Sequence-based reagent | Genotyping PCR primer forDbx1tm2(cre)Apie | Microsynth (desalted;100 µM stock) | Mut-F (cre83): GTC CAA TTT ACT GAC CGT ACA CC | |
| Sequence-based reagent | Genotyping PCR primer forDbx1tm2(cre)Apie | Microsynth (desalted;100 µM stock) | Mut-R (cre85): GTT ATT CGG ATC ATC AGC TAC ACC | |
| Commercial assay or kit | VECTASTAIN Elite ABC-HRP Kit | VectorLab, Burlingame, CA | PK-6100 | Manufacturer's protocol |
| Commercial assay or kit | DAB Peroxidase (HRP) Substrate Kit (with Nickel), 3,3’-diaminobenzidine | VectorLab | SK-4100 | Manufacturer's protocol |
| Commercial assay or kit | RNAscope Fluorescence Multiplex Reagent Kit | Advanced Cell Diagnostics, Newark, CA | 320850 | Manufacturer's protocol (fresh frozen tissue) |
| Chemical compound, drug | Nε-(+)-Biotinyl-L-lysine (biocytin) | Sigma Aldrich, Germany | B4261 | Used at 8.1 mM |
| Chemical compound, drug | Fast Red tablets | Kem-En-Tech, Denmark | 4210 | Manufacturer's protocol |
| Chemical compound, drug | Hoechst 33258 | Sigma Aldrich | 23491-45-4 | (1:10000) |
| Chemical compound, drug | Thiopental Inresa 0.5 g | Inresa Arzneimittel GmbH, Germany | Used at 50 mg/kg | |
| Software, algorithm | Microsoft Office 2017 (Excel, Word) | © 2017 Microsoft, Redmond, WA | v.16.9.1 | Manuscript editing |
| Software, algorithm | Adobe Suit CC (Photoshop, Illustrator, Acrobat) | Adobe Systems, San José, CA | v.22.0.1 | Image treatment, figure editing |
| Software, algorithm | GraphPad Prism | GraphPad software, Inc., La Jolla, CA | v.7.0 | Statistics, graph editing |
| Software, algorithm | Fiji | doi:10.1038/nmeth.2019 | v.2.0.0 | Image editing, manual counting |
| Software, algorithm | EndNote X | Thomson Reuters, Canada | v.7.7.1 | Reference editing, bibliography |
| Software, algorithm | Neurolucida v.11.02.1 | Microbrightfield, MBF Bioscience, Williston, VT | v.11.02.1 | Neuron reconstruction |
| Software, algorithm | Neurolucida Explorer | Microbrightfield, MBF Bioscience | v.11.02.1 | Morphological reconstruction editing |
| Software, algorithm | MatLab | MathWorks, Natick, MA | Electrophysiological recordings measurment/editing | |
| Software, algorithm | Ephus (MATLAB-based) | doi: 10.3389/fncir.2010.00100 | v. 2.1.0 | Electrophysiological recordings data aquisition |
| Software, algorithm | Clampfit | Molecular Devices, San José, CA | v. 10.1.0.10 | Electrophysiological recordings offline analysis |
| Software, algorithm | R programming language | www.R-project.org | v. 3.4.0 | Statistics |
| Software, algorithm | R package bmrm | doi:10.1038/ncomms14219 | v. 3.5 | Prediction model |
| Software, algorithm | NLMorpholoyConverter | http://neuronland.org/NLMorphologyConverter/NLMorphologyConverter.html | v. 0.8.1 | Morphological reconstruction editing |
| Software, algorithm | R package NeuroAnatomy Toolbox | doi: 10.1016/j.neuron.2016.06.012 | v. 1.8.12.9000 | Morphological reconstruction editing |
Animals
Animal experiments were approved by the local Geneva animal care committee (GE113/16) and conducted according to international and Swiss guidelines. Mice were housed in the conventional area of the animal facility of the Geneva Medical Center. Water and food were provided ad libitum and both temperature (22 ± 2°C) and dark/light cycles (12 hr each) were controlled. Timed-pregnant mice were obtained by overnight mating and the following morning was counted as embryonic day (E) E0.5. Tg(Htr3a-EGFP)DH30Gsat mice expressing the enhanced GFP under the control of the Htr3a regulatory sequences (Htr3a-GFP) were provided by the GENSAT Consortium and maintained on a C57Bl/6 background (Murthy et al., 2014). B6.Cg-Gt(ROSA)26Sortm14(CAG-tdTomato)Hze/J loxP flanked reporter mice (R26R-tdTOMfl/fl) were obtained from Jackson Laboratory. Htr3a-GFP mice were crossed with R26R-tdTOMfl/fl mice to obtain Htr3a-GFP; R26R-tdTOMfl/fl mice. Tg(Hmx3-icre)1Kess (Hmx3-Cre) mice were obtained from Oscar Marin and previously described (Gelman et al., 2009). Dbx1tm2(cre)Apie (Dbx1-Cre) mice were obtained from Alessandra Pierani and previously described (Gelman et al., 2011). Details of the genotyping procedure are given in the Key Resources Table.
Tissue processing and immunohistochemistry
Request a detailed protocolPregnant females were euthanized by lethal intraperitoneal (i.p.) injection of pentobarbital (50 mg/kg), embryos were collected by caesarian cut and brains dissected and fixed overnight (O.N.) in cold 4% paraformaldehyde dissolved in 0.1M phosphate buffer (PFA) pH 7.4. For postnatal brains, animals were deeply anesthetized by i.p. injection of pentobarbital and transcardially perfused with 0.9% saline/liquemin followed by cold 4% PFA. Brains were cut on a Vibratome (Leica VT1000S) at 60 µm for immunohistochemistry (IHC) or at 80–100 µm for free-floating in situ hybridization (ISH). Sections were kept in a cryoprotective solution at −20°C or processed directly for IHC or ISH as described (Murthy et al., 2014). The following primary antibodies were used: rabbit anti-GFAP (1:2000, Abcam), goat anti-GFP (1:2000, Abcam), rabbit anti-GFP (1:500, Millipore), mouse anti-NeuN (1:500, Millipore), rabbit anti-NKX2.1 (1:100, Santa Cruz Biotechnology), rabbit anti-NPY (1:500, Abcam), rabbit anti-NR2F2 (1:500, Abcam), goat anti-PROX1 (1:250, R and D System), mouse anti-Parvalbumin (PV) (1:2000, Swant), mouse anti-Reelin (1:500, Abcam), rabbit anti-S100β (1:2000, Abcam), rat anti-Somatostatin (SST) (1:500, Millipore), rabbit anti-SOX6 (1:500, Abcam), goat anti-SOX10 (1:100, Santa Cruz Biotechnology), goat anti-SP8 (1:50, Santa Cruz Biotechnology), goat anti-tdTomato (tdTOM) (1:500, Sicgen), rabbit anti-VIP (1:500, Abcam). Secondary goat or donkey Alexa-405,–488, −568 and −647 antibodies (Abcam, Invitrogen) raised against the appropriate species were used at a dilution of 1:500 and sections were counterstained with Hoechst 33258 (1:10000) when no Alexa-405 staining was done. A list of the antibodies is given in the Key Resources Table.
In situ hybridization and RNAscope
Request a detailed protocolSections were hybridized with the respective DIG-labeled RNA probes as described previously (Murthy et al., 2014). The Htr3a plasmid probe was linearized with HindIII-HF for antisense RNA probe synthesis by T7 polymerase (kind gift from Dr. B. Emerit). The Lhx6 plasmid probe (Liodis et al., 2007) was linearized with Not1 for antisense RNA probe synthesis by T3 polymerase (kind gift from Dr. M. Denaxa). The unbound probe was washed and slices incubated with alkaline phosphatase-conjugated anti-DIG antibody (1:2000, Roche) O.N. at 4°C. Fast Red (Kem-En-Tech) was used as an alkaline phosphatase fluorescent substrate to reveal the hybridized probe. We took advantage of the removal of both GFP and tdTOM endogenous fluorescence due to protocol treatments and revealed them by IHC using green and far-red emitting secondary antibodies, respectively. For illustration purposes, the bound probe (red) and the tdTOM (far red) are shown in blue and red, respectively. For RNAscope experiments, P30 brains were rapidly extracted and fresh frozen. After dehydration and protease treatment, coronal 12 µm-thick brain sections were processed using the RNAscope Multiplex Fluorescent Reagent Kit (Advanced Cell Diagnostics) according to the manufacturer’s protocol. Probes targeting mRNAs of the GFP and tdTOM transgenes and of the endogenous Car4 gene were designed by Advanced Cell Diagnostics.
Imaging and quantification of interneuron identity and distribution
Request a detailed protocolImages were acquired using confocal microscopes (Nikon A1R or Axio Imager.Z2 Basis LSM 800) equipped with oil-immersion 40x, 60x and 63x objectives (CFI Plan Fluor 40x/1.3 and CFI Plan Apo VC H 60x/1.4, Nikon or Plan-APO (UV) VIS-IR 40x/1.4 and Plan-Apochromat f/ELYRA 63x/1.4, LSM). For widefield illustrations (Figure 2—figure supplement 1), images were taken with Axioscan.Z1 slidescanner (Zeiss), equipped with Plan-Apochromat 10x/0.45 objective (Zeiss). Images were lightly treated (gamma, brightness and and despeckle filter only) for visual purpose with Photoshop CC and manual counts were achieved with Fiji. Data are presented as brain averages calculated from at least three slices at different rostro-caudal levels per brain (except for P5 Dbx1 brain 3). A detailed description of the counts, cells and brains in the different experiments is given in Supplementary file 1.
Electrophysiological recordings and morphological tracing
Request a detailed protocol300 µm-thick coronal brain slices were prepared from 3 to 4 weeks old Hmx3; tdTOM+/Htr3a-GFP+ mice with a vibratome (Leica VT 1000S). In the recording chamber, slices were continuously superfused with ACSF (32°C) containing (in mM): NaCl (119), KCl (2.5), CaCl2 (2.5), MgSO4 (1.3), NaH2PO4 (1.0), NaHCO3 (26.2), and glucose (22), and equilibrated with 95% O2/5% CO2, pH 7.4. Whole-cell recordings were obtained from visually identified Hmx3; tdTOM+/Htr3a-GFP+ in cortical layers 1–6 and Hmx3; tdTOM-/Htr3a-GFP+ INs in L1, using an upright microscope (Zeiss Axioskop FS) equipped with differential interference contrast and standard epifluorescence. Borosilicate glass patch pipettes had a resistance of 5–6 MΩ when filled with an internal solution containing (in mM): K gluconate (135), KCl (4), HEPES (10), Phosphocreatine (10), Mg-ATP (4), Na-GTP (0.3), and biocytin (8.1). Current clamp recordings were performed at rest and firing properties were studied by delivering consecutive current pulses, 500 ms duration each, ranging from −20 to +360 pA with a 5 pA increment, every 3 s. Data were acquired using a Multiclamp 700B Amplifier (Molecular Devices), and digitized at 10 kHz (National Instruments), using MATLAB (MathWorks)-based Ephus software (Ephus; The Janelia Farm Research Center). Offline analysis was performed using Clampfit (Version 10.1.0.10, Molecular Devices). Cells were accepted for analysis only if their series resistance was below 30 MΩ and did not change more than 20% during recordings. Following patch-clamp recordings, slices were incubated in ACSF for 1–2 hr at room temperature, then fixed overnight with 4% PFA / 2% Glutaraldehyde in 0.1 M phosphate buffer. Biocytin-filled recorded cells were revealed with IHC, using streptavidin-Alexa 647 conjugate (1:500, Thermo Fisher Scientific), and confirmed being in L1 and expressing Hmx3; tdTOM and/or Htr3a-GFP. For detailed morphology, slices were quenched for endogenous peroxidase activity in methanol/0.5% H202, blocked in 0.05 M Tris buffer pH 7.4/0.6% NaCl/0.3% Triton X-100/10% normal horse serum (NHS) and incubated (O.N., 4°C) with avidin-biotin complex (Vectastain Elite ABC-HRP Kit) in 0.1M Tris buffer pH 7.7. 3,3’-diaminobenzidine (DAB) revelation was performed following the manufacturer’s protocol (Vectastain DAB Kit; SK-4100). Slices were finally dehydrated in graded series of ethanol/xylene and mounted in Eukitt (Sigma). Morphological reconstructions of biocytin-filled cells were performed with Neurolucida software (v. 11.02.1, MBF Bioscience, Microbrightfield), linked to a microscope (Nikon eclipse 80i) equipped with an oil-immersion 100x objective (Plan Apo VC/1.4, Nikon). Brightfield images of the reconstructed cells were acquired with the same microscope and a 10x objective (Plan Apo/0.45, Nikon). Traces were extracted with Neurolucida Explorer (v.11.02.1, MBF Bioscience, Microbrightfield). 14 Hmx3; tdTOM+/Htr3a-GFP+ cells (7 in L1, 5 cells in L2/3 and 2 cells in L5) and 3 Hmx3; tdTOM-/Htr3a-GFP+ INs (3 in L1) from four brains were recovered for morphology. For L1 cells, border artefacts in morphological tracings due to tissue compression were corrected. Traces from 14 Hmx3; tdTOM+/Htr3a-GFP+ and 3 Hmx3; tdTOM-/Htr3a-GFP+ INs from four brains were analysed blindly. The membrane resistance (Rm), the membrane resting potential and five properties of the first action potential (AP) at rheobase - i) threshold potential (Vth); ii) AP amplitude (peak); iii) AP latency from current step onset (delay); iv) after-hyperpolarization potential amplitude (AHP) and when present; v) after-depolarization potential amplitude (ADP) - were measured for all recorded cells. Both electrophysiological features and morphological tracings were analyzed blindly and data were attributed back to their corresponding cell. Values for each recorded cell are provided in Figure 6—source data 2 and Figure 6—figure Supplement 3—source data 1.
Statistical analysis and prediction model
Request a detailed protocolAnimals were used regardless of their sex and statistical analysis was done with R programming language and GraphPad Prism. No statistics were used to determine optimal group sample size; however, sample sizes were similar to those used in previous publications from our group and others. Normality of the samples was assessed with D’Agostino-Pearson test and when distribution was not normal, non-parametric tests were applied. Using bmrm (v3.3) package for L1-regularized logistic regression model, data were standardized, and a L1-regularized logistic regression model was trained to distinguish between Htr3a-GFP+ INs that were Hmx3-derived and those which were not. This model assigned a linear weight that reflects the power of each feature in the model logistic regression and computed a probability that a given cell is Hmx3-derived in such a way that the misclassification error on the training data was minimized (Figure 6I–J). Classification performance of the L1-regularized logistic regression algorithm was assessed by leave-one-out-cross-validation (LOOCV). It consists in training a model on all but one cell, feeding the model with this isolated cell to predict its origin and finally assessing if the prediction is correct. Looping it over all cells, yields a prediction value for each cell, which is used to estimate the generalization error of the classifier. Finally, in order to determine if the prediction made by the logistic regression model improved over the signal contained into each feature taken individually, receiver operating characteristic (ROC) curves were drawn to visualize the sensitivity/specificity ratio for each feature and for the leave-one-out predictions. Areas under the curves (AUC) were analyzed to determine the strongest signals (Figure 6—figure supplement 2, Figure 6—source data 2).
References
-
Decoding the transcriptional basis for GABAergic interneuron diversity in the mouse neocortexEuropean Journal of Neuroscience 34:1542–1552.https://doi.org/10.1111/j.1460-9568.2011.07904.x
-
Basket cell dichotomy in microcircuit functionThe Journal of Physiology 590:683–694.https://doi.org/10.1113/jphysiol.2011.223669
-
SOX6 controls dorsal progenitor identity and interneuron diversity during neocortical developmentNature Neuroscience 12:1238–1247.https://doi.org/10.1038/nn.2387
-
The emerging role of GABAB receptors as regulators of network dynamics: fast actions from a 'slow' receptor?Current Opinion in Neurobiology 26:15–21.https://doi.org/10.1016/j.conb.2013.10.002
-
NKX2.1 specifies cortical interneuron fate by activating Lhx6Development 135:1559–1567.https://doi.org/10.1242/dev.015123
-
Mechanisms of inhibition within the telencephalon: "where the wild things are"Annual Review of Neuroscience 34:535–567.https://doi.org/10.1146/annurev-neuro-061010-113717
-
A wide diversity of cortical GABAergic interneurons derives from the embryonic preoptic areaJournal of Neuroscience 31:16570–16580.https://doi.org/10.1523/JNEUROSCI.4068-11.2011
-
The embryonic preoptic area is a novel source of cortical GABAergic interneuronsJournal of Neuroscience 29:9380–9389.https://doi.org/10.1523/JNEUROSCI.0604-09.2009
-
The organization of two new cortical interneuronal circuitsNature Neuroscience 16:210–218.https://doi.org/10.1038/nn.3305
-
Genetic programs controlling cortical interneuron fateCurrent Opinion in Neurobiology 26:79–87.https://doi.org/10.1016/j.conb.2013.12.012
-
Serotonin receptor 3A controls interneuron migration into the neocortexNature Communications 5:5524.https://doi.org/10.1038/ncomms6524
-
Three groups of interneurons account for nearly 100% of neocortical GABAergic neuronsDevelopmental Neurobiology 71:45–61.https://doi.org/10.1002/dneu.20853
-
RNAscope: a novel in situ RNA analysis platform for formalin-fixed, paraffin-embedded tissuesThe Journal of Molecular Diagnostics : JMD 14:22–29.https://doi.org/10.1016/j.jmoldx.2011.08.002
-
The origin and specification of cortical interneuronsNature Reviews Neuroscience 7:687–696.https://doi.org/10.1038/nrn1954
-
Fate mapping Nkx2.1-lineage cells in the mouse telencephalonThe Journal of Comparative Neurology 506:16–29.https://doi.org/10.1002/cne.21529
Article and author information
Author details
Funding
Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung (Synapsy 51NF40-158776; 31003A_153448 and CRSII3_154453)
- Anthony Holtmaat
Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung (Synapsy 51NF40-158776 and 31003A_155896/1)
- Alexandre Dayer
International Foundation for Research in Paraplegia (Chair Alain Rossier)
- Alexandre Dayer
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Acknowledgements
We thank Oscar Marin and Nicoletta Kessaris for providing the Hmx3-Cre mice and Alessandra Pierani for providing the Dbx1-Cre mice. This work was supported by a Swiss National Foundation (SNF) Synapsy grant (51NF40-158776) and a SNF (31003A_155896/1) grant for AD.
Ethics
Animal experimentation: Animal experiments were approved by the local Geneva animal care committee and conducted according to international and Swiss guidelines.(GE113/16)
Copyright
© 2018, Niquille et al.
This article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use and redistribution provided that the original author and source are credited.
Metrics
-
- 4,939
- views
-
- 653
- downloads
-
- 50
- citations
Views, downloads and citations are aggregated across all versions of this paper published by eLife.
Citations by DOI
-
- 50
- citations for umbrella DOI https://doi.org/10.7554/eLife.32017






