Research: Adequate statistical power in clinical trials is associated with the combination of a male first author and a female last author
Abstract
Clinical trials have a vital role in ensuring the safety and efficacy of new treatments and interventions in medicine. A key characteristic of a clinical trial is its statistical power. Here we investigate whether the statistical power of a trial is related to the gender of first and last authors on the paper reporting the results of the trial. Based on an analysis of 31,873 clinical trials published between 1974 and 2017, we find that adequate statistical power was most often present in clinical trials with a male first author and a female last author (20.6%, 95% confidence interval 19.4-21.8%), and that this figure was significantly higher than the percentage for other gender combinations (12.5-13.5%; P<0.0001). The absolute number of female authors in clinical trials gradually increased over time, with the percentage of female last authors rising from 20.7% (1975-85) to 28.5% (after 2005). Our results demonstrate the importance of gender diversity in research collaborations and emphasize the need to increase the number of women in senior positions in medicine.
https://doi.org/10.7554/eLife.34412.001Introduction
There is increasing awareness that many clinical trials have systematic methodological flaws and that their results may be biased, exaggerated, and difficult to reproduce (Ioannidis et al., 2014). Clinical trials are the result of complex group efforts. Male and female researchers differ in their collaborative strategies which depends on the level of their expertise and whether they have a junior or senior position (Zeng et al., 2016; Bozeman and Gaughan, 2011). There are indications that mixed gender teams may make the best use of personal knowledge and skills, (Nielsen et al., 2017) an effect also reported in a scientific research context (Woolley et al., 2010; Campbell et al., 2013). Even though this may in turn positively influence the quality of clinical research, (Nielsen et al., 2017) no studies have systematically investigated whether collaborations between male and female researchers affect the quality of clinical trials. This topic is important in light of the existing diversity challenges that currently exist in the biomedical research field (Valantine and Collins, 2015).
In this study, we therefore aimed to quantify the effect of collaborations across gender combinations of junior and senior authors on the methodological quality of clinical trials. To this aim, we determined the percentage of adequately powered trials in 31,873 clinical trials published between 1974 and 2017 based on Cochrane meta-analyses. As statistical power reflects the chance of detecting a true effect, it is regarded as one of the key elements of responsible research (Button et al., 2013) and considered essential in reproducible clinical research (Halpern et al., 2002). We found that the probability of having adequate statistical power for one combination - male first author, female last author - was significantly higher than that for the other three possible combinations. Moreover, this effect was present across countries and most medical fields.
Results
Statistical power and gender combinations in all clinical trials (N=31,873)
In our 31,873 trials, the number of published clinical trials with adequate statistical power (>80%) was generally low (12-13%; Figure 1A, left panel). The exception was the set of trials with a male first author combined with a female last author with 20.6% of outcomes adequately powered (CI 19.4–21.8). This percentage was significantly higher in comparison to the three other combinations (highest odds ratio 2.08, CI 1.87–2.30, P<0.0001). Cut-off values for adequate power set to either 70% or 90% yielded comparable results (P<0.0001; Figure 1B). The percentage of adequately powered trials in which the gender combination was unknown was 13.8% (CI 13.6–14.1; Figure 1C). Irrespective of the gender of the first author, clinical trials with female last authors had a higher statistical power compared to male last authors: 16.6% (CI 15.9–17.4) versus 12.9% (CI 12.6–13.3; Figure 2). The average statistical power of clinical trials with missing gender was comparable to those with known gender combinations (Figures 1C and 2). Slightly higher odds for adequately powered trials were also found in the author combination ‘both males’ and ‘female – male (last)’ in comparison to the reference group ‘both females’: odds ratios 1.28 (CI 1.17–1.41, P<0.0001) and 1.25 (CI 1.13–1.39, P<0.0001), respectively (Table 1). In the sensitivity analysis model estimates were slightly lower (relative estimate difference 2.3% to 4.8%; Table 2).
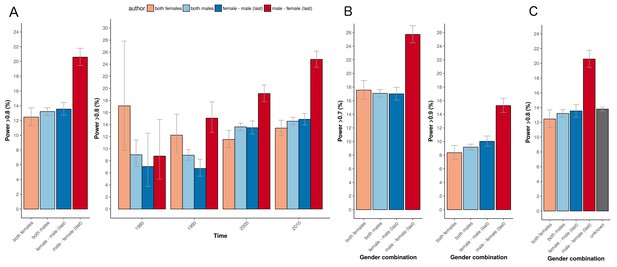
Percentage of adequately powered trials for the four different gender combinations of first and last author.
(A) Percentage of trials with power > 0.8 published between 1974 and 2017 for the four gender combinations (left panel) and for four periods (1975–1985; 1985–1995; 1995–2005; >2005) during this time (right panel). (B) Percentage of trials published with power > 0.7 (left) and power > 0.9 (right) for the four gender combinations. (C) Percentage of trials with power > 0.8 for the four gender combinations, including the trials were gender could not be determined for the first and/or last author (‘unknown’). Error bars represent the 95% confidence interval for proportions for all panels.
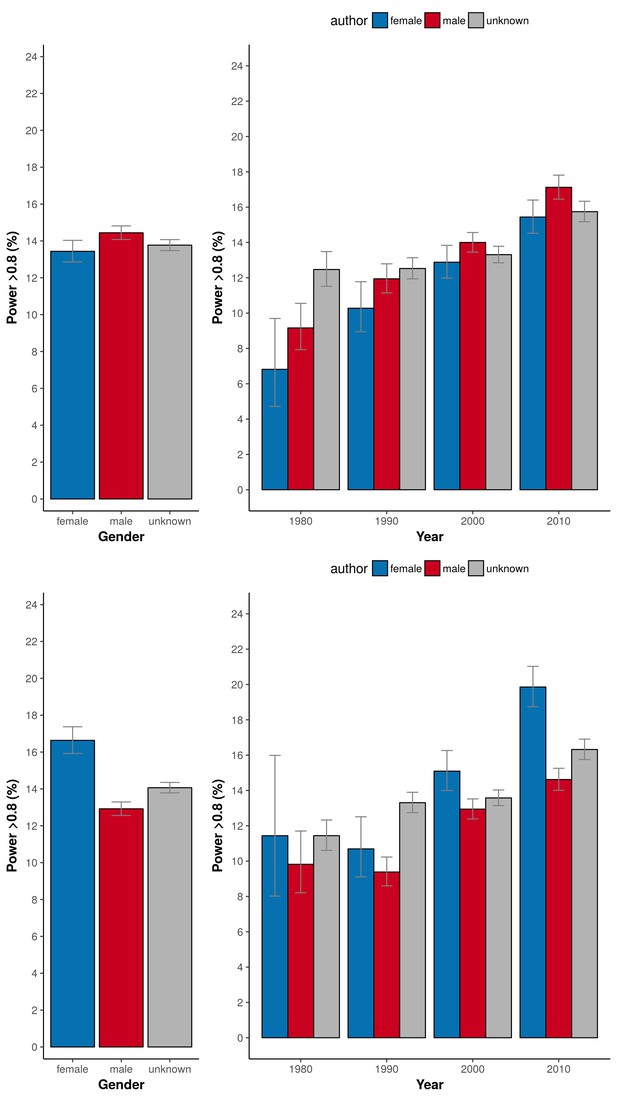
Percentage of adequately powered trials when the gender of the first and last author is male, female or unknown.
Left: Percentage of trials with power > 0.8 plotted for the gender of the first author (top) and the last author (bottom). Right: Percentage of trials with power > 0.8 plotted for four periods (1975–1985; 1985–1995; 1995–2005; >2005) for the gender of the first author (top) and the last author (bottom). Error bars represent the 95% confidence interval for proportions for all panels.
Model estimates for the variables fitted against adequately powered trials.
https://doi.org/10.7554/eLife.34412.004| Variables | Odds ratio | 95% CI | Z value | P value | |
|---|---|---|---|---|---|
| Author combination | |||||
| Both females | 1.00 (ref.) | ||||
| Both males | 1.28 | 1.17 | 1.41 | 5.22 | <0.0001 |
| Female - male (last) | 1.25 | 1.13 | 1.39 | 4.29 | <0.0001 |
| Male - female (last) | 2.08 | 1.87 | 2.30 | 13.94 | <0.0001 |
| Time | |||||
| Publication year | 1.03 | 1.02 | 1.03 | 12.05 | <0.0001 |
| Country group | |||||
| Anglosphere | 1.00 (ref.) | ||||
| Europe | 0.76 | 0.71 | 0.81 | 8.87 | <0.0001 |
| Non-western | 0.87 | 0.80 | 0.94 | 3.69 | <0.0001 |
| Medical discipline | |||||
| Allergy & intolerance | 1.00 (ref) | ||||
| Blood disorders | 0.45 | 0.34 | 0.62 | 5.11 | <0.0001 |
| Child health | 0.47 | 0.36 | 0.61 | 5.68 | <0.0001 |
| Complementary medicine | 0.23 | 0.17 | 0.31 | 9.14 | <0.0001 |
| Consumer strategies | 0.66 | 0.41 | 1.03 | 1.80 | 0.072 |
| Dentistry & oral health | 1.05 | 0.68 | 1.59 | 0.21 | 0.832 |
| Developmental problems | 0.69 | 0.47 | 1.00 | 1.98 | 0.048 |
| Ear, nose & throat | 0.37 | 0.24 | 0.55 | 4.77 | <0.0001 |
| Effective health systems | 0.75 | 0.53 | 1.07 | 1.57 | 0.115 |
| Endocrine & metabolic | 0.29 | 0.20 | 0.42 | 6.51 | <0.0001 |
| Eyes & vision | 0.56 | 0.38 | 0.81 | 3.02 | 0.003 |
| Gastroenterology & hepatology | 0.49 | 0.38 | 0.65 | 5.07 | <0.0001 |
| Genetic disorders | 0.19 | 0.12 | 0.30 | 7.09 | <0.0001 |
| Gynaecology | 0.69 | 0.52 | 0.92 | 2.58 | 0.01 |
| Health & safety at work | 0.24 | 0.13 | 0.42 | 4.74 | <0.0001 |
| Heart & circulation | 0.29 | 0.22 | 0.39 | 8.24 | <0.0001 |
| Infectious disease | 0.61 | 0.47 | 0.80 | 3.62 | <0.0001 |
| Kidney disease | 0.80 | 0.58 | 1.12 | 1.28 | 0.201 |
| Lungs & airways | 0.35 | 0.27 | 0.46 | 7.56 | <0.0001 |
| Mental health | 0.53 | 0.40 | 0.71 | 4.40 | <0.0001 |
| Neonatal care | 0.47 | 0.34 | 0.64 | 4.68 | <0.0001 |
| Neurology | 0.56 | 0.42 | 0.74 | 4.08 | <0.0001 |
| Orthopaedics & trauma | 0.79 | 0.60 | 1.05 | 1.63 | 0.103 |
| Pain & anaesthesia | 0.64 | 0.49 | 0.84 | 3.23 | 0.001 |
| Pregnancy & childbirth | 0.58 | 0.44 | 0.77 | 3.76 | <0.0001 |
| Public health | 1.23 | 0.78 | 1.92 | 0.89 | 0.372 |
| Rheumatology | 0.75 | 0.57 | 1.00 | 2.02 | 0.043 |
| Skin disorders | 0.89 | 0.65 | 1.23 | 0.69 | 0.488 |
| Tobacco, drugs & alcohol | 0.34 | 0.26 | 0.46 | 7.39 | <0.0001 |
| Urology | 1.04 | 0.74 | 1.45 | 0.21 | 0.834 |
| Wounds | 0.36 | 0.21 | 0.61 | 3.73 | <0.0001 |
Model estimates from the sensitivity analysis (with individual countries) for the variables fitted against adequately powered trials.
https://doi.org/10.7554/eLife.34412.005| Variables | Odds ratio | 95% CI | Z value | P value | |
|---|---|---|---|---|---|
| Author combination | |||||
| Both females | 1.00 (ref.) | ||||
| Both males | 1.25 | 1.13 | 1.37 | 4.58 | <0.001 |
| Female - male (last) | 1.19 | 1.07 | 1.32 | 3.28 | 0.001 |
| Male - female (last) | 1.98 | 1.78 | 2.19 | 12.95 | <0.001 |
| Time | |||||
| Publication year | 1.02 | 1.02 | 1.03 | 14.5 | <0.001 |
| Country | |||||
| Argentina | 1.00 (ref.) | ||||
| Australia | 0.79 | 0.52 | 1.19 | 1.12 | 0.261 |
| Austria | 1.31 | 0.84 | 2.02 | 1.19 | 0.232 |
| Bangladesh | 3.29 | 2.00 | 5.41 | 4.69 | <0.001 |
| Belgium | 0.94 | 0.61 | 1.45 | 0.29 | 0.775 |
| Brazil | 0.98 | 0.63 | 1.51 | 0.10 | 0.92 |
| Canada | 1.16 | 0.78 | 1.72 | 0.72 | 0.474 |
| Chile | 0.74 | 0.39 | 1.42 | 0.89 | 0.371 |
| China | 1.20 | 0.8 | 1.81 | 0.87 | 0.383 |
| Colombia | 1.95 | 1.17 | 3.26 | 2.55 | 0.011 |
| Costa Rica | 0.00 | 0.00 | Inf | 0.14 | 0.891 |
| Croatia | 0.47 | 0.22 | 1.03 | 1.88 | 0.06 |
| Czech Republic | 0.71 | 0.45 | 1.13 | 1.45 | 0.147 |
| Denmark | 1.24 | 0.82 | 1.87 | 1.03 | 0.303 |
| Egypt | 1.78 | 1.13 | 2.79 | 2.50 | 0.013 |
| Finland | 0.88 | 0.58 | 1.32 | 0.63 | 0.527 |
| France | 0.91 | 0.61 | 1.37 | 0.44 | 0.663 |
| Gambia | 1.05 | 0.56 | 1.99 | 0.16 | 0.87 |
| Germany | 0.90 | 0.6 | 1.34 | 0.53 | 0.593 |
| Ghana | 0.84 | 0.48 | 1.48 | 0.61 | 0.544 |
| Greece | 0.46 | 0.29 | 0.75 | 3.12 | 0.002 |
| Hong Kong | 1.37 | 0.89 | 2.11 | 1.44 | 0.15 |
| Hungary | 2.87 | 1.75 | 4.7 | 4.18 | <0.001 |
| India | 0.89 | 0.58 | 1.35 | 0.56 | 0.573 |
| Indonesia | 0.71 | 0.34 | 1.48 | 0.93 | 0.354 |
| Iran | 1.14 | 0.73 | 1.79 | 0.59 | 0.557 |
| Ireland | 0.80 | 0.49 | 1.32 | 0.87 | 0.387 |
| Israel | 0.80 | 0.51 | 1.26 | 0.98 | 0.328 |
| Italy | 1.03 | 0.69 | 1.53 | 0.15 | 0.881 |
| Japan | 0.35 | 0.22 | 0.53 | 4.83 | <0.001 |
| Jordan | 3.91 | 2.09 | 7.32 | 4.27 | <0.001 |
| Kenya | 0.42 | 0.18 | 1.00 | 1.97 | 0.049 |
| Korea | 1.56 | 1.02 | 2.39 | 2.07 | 0.038 |
| Lebanon | 1.36 | 0.75 | 2.46 | 1.01 | 0.311 |
| Malawi | 0.12 | 0.03 | 0.52 | 2.83 | 0.005 |
| Malaysia | 0.78 | 0.34 | 1.79 | 0.59 | 0.552 |
| Mali | 0.75 | 0.29 | 1.91 | 0.61 | 0.543 |
| Mexico | 1.07 | 0.62 | 1.85 | 0.25 | 0.8 |
| Netherlands | 0.71 | 0.47 | 1.07 | 1.62 | 0.106 |
| New Zealand | 1.28 | 0.76 | 2.14 | 0.94 | 0.349 |
| Nigeria | 1.32 | 0.70 | 2.48 | 0.87 | 0.386 |
| Norway | 0.89 | 0.56 | 1.41 | 0.49 | 0.624 |
| Pakistan | 0.93 | 0.48 | 1.83 | 0.20 | 0.844 |
| Papua New Guinea | 0.00 | 0.00 | Inf | 0.10 | 0.918 |
| Peru | 0.99 | 0.57 | 1.7 | 0.04 | 0.967 |
| Poland | 0.39 | 0.22 | 0.68 | 3.29 | 0.001 |
| Portugal | 3.17 | 1.84 | 5.45 | 4.17 | <0.001 |
| Qatar | 0.00 | 0.00 | Inf | 0.11 | 0.916 |
| Saudi Arabia | 0.54 | 0.30 | 0.98 | 2.02 | 0.043 |
| Singapore | 1.14 | 0.68 | 1.93 | 0.50 | 0.614 |
| Slovenia | 0.00 | 0.00 | Inf | 0.11 | 0.91 |
| South Africa | 1.24 | 0.79 | 1.96 | 0.93 | 0.355 |
| Spain | 1.08 | 0.71 | 1.62 | 0.35 | 0.73 |
| Sweden | 1.24 | 0.83 | 1.85 | 1.03 | 0.301 |
| Switzerland | 0.66 | 0.43 | 1.02 | 1.89 | 0.059 |
| Taiwan | 0.45 | 0.29 | 0.71 | 3.43 | 0.001 |
| Thailand | 1.53 | 0.99 | 2.37 | 1.93 | 0.053 |
| Turkey | 0.64 | 0.42 | 0.98 | 2.06 | 0.039 |
| Uganda | 1.27 | 0.56 | 2.88 | 0.58 | 0.56 |
| UK | 1.25 | 0.84 | 1.85 | 1.10 | 0.273 |
| USA | 1.42 | 0.96 | 2.10 | 1.78 | 0.076 |
| Venezuela | 5.25 | 3.22 | 8.54 | 6.67 | <0.001 |
| Vietnam | 0.00 | 0.00 | Inf | 0.12 | 0.907 |
| Zimbabwe | 1.93 | 0.90 | 4.12 | 1.70 | 0.089 |
| Other countries | 0.75 | 0.48 | 1.17 | 1.28 | 0.201 |
| Medical discipline | |||||
| Allergy & intolerance | 1.00 (ref.) | ||||
| Blood disorders | 0.49 | 0.39 | 0.63 | 5.79 | <0.001 |
| Child health | 0.55 | 0.45 | 0.67 | 5.87 | <0.001 |
| Complementary medicine | 0.26 | 0.20 | 0.33 | 11.21 | <0.001 |
| Consumer strategies | 0.94 | 0.66 | 1.34 | 0.35 | 0.73 |
| Dentistry & oral health | 1.43 | 1.07 | 1.92 | 2.41 | 0.016 |
| Developmental problems | 0.78 | 0.58 | 1.05 | 1.64 | 0.101 |
| Ear, nose & throat | 0.51 | 0.39 | 0.68 | 4.66 | <0.001 |
| Effective health systems | 0.85 | 0.63 | 1.14 | 1.11 | 0.269 |
| Endocrine & metabolic | 0.4 | 0.30 | 0.53 | 6.58 | <0.001 |
| Eyes & vision | 0.51 | 0.38 | 0.70 | 4.27 | <0.001 |
| Gastroenterology & hepatology | 0.56 | 0.46 | 0.69 | 5.41 | <0.001 |
| Genetic disorders | 0.29 | 0.20 | 0.42 | 6.51 | <0.001 |
| Gynaecology | 0.82 | 0.66 | 1.01 | 1.84 | 0.066 |
| Health & safety at work | 0.54 | 0.37 | 0.79 | 3.16 | 0.002 |
| Heart & circulation | 0.34 | 0.27 | 0.43 | 9.43 | <0.001 |
| Infectious disease | 0.8 | 0.65 | 0.99 | 2.09 | 0.036 |
| Kidney disease | 0.71 | 0.55 | 0.92 | 2.59 | 0.01 |
| Lungs & airways | 0.47 | 0.38 | 0.58 | 7.04 | <0.001 |
| Mental health | 0.6 | 0.48 | 0.75 | 4.58 | <0.001 |
| Neonatal care | 0.38 | 0.29 | 0.48 | 7.81 | <0.001 |
| Neurology | 0.7 | 0.57 | 0.87 | 3.26 | 0.001 |
| Orthopaedics & trauma | 1.18 | 0.96 | 1.46 | 1.56 | 0.12 |
| Pain & anaesthesia | 0.73 | 0.60 | 0.90 | 2.92 | 0.003 |
| Pregnancy & childbirth | 0.69 | 0.55 | 0.85 | 3.40 | 0.001 |
| Public health | 1.72 | 1.24 | 2.37 | 3.27 | 0.001 |
| Rheumatology | 0.97 | 0.78 | 1.20 | 0.31 | 0.757 |
| Skin disorders | 1.26 | 0.99 | 1.59 | 1.89 | 0.058 |
| Tobacco. drugs & alcohol | 0.4 | 0.32 | 0.50 | 7.97 | <0.001 |
| Urology | 1.27 | 1.00 | 1.63 | 1.92 | 0.054 |
| Wounds | 0.8 | 0.59 | 1.08 | 1.44 | 0.15 |
Trends across countries
The world map in Figure 3 shows the geographical distribution of the trials in our sample (based on affiliation of the first author). The percentage of trials originating from Anglosphere countries (United States, United Kingdom, Canada, Australia and New Zealand) was 46.9%; the percentage from European countries was 32.9%; and the percentage from non-western countries was 20.2% (with the top five being Turkey, Japan, India, China and Israel). European trials had lower odds of adequate statistical power compared to Anglosphere trials (odds ratio: 0.76, CI 0.71–0.81, P<0.0001; Figure 4A). This was also the case in trials from Non-western countries (odds ratio: 0.87, CI 0.80–0.94, P<0.0001; Table 1). Individual country data, from the sensitivity analysis, is provided in Table 2.
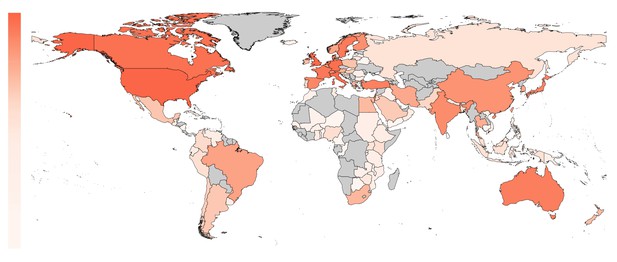
The proportion of included trials mapped per country on a white to red color scale (range: 0 – 24%).
The highest proportion of first authors were affiliated with an institution in the United States. Countries not present in any affiliation are plotted in gray.
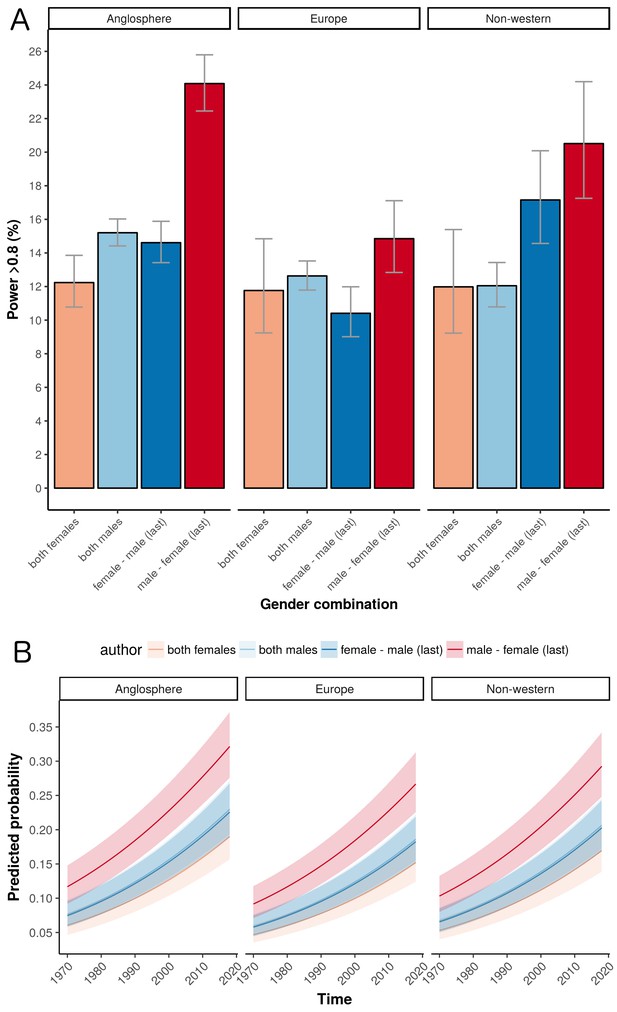
The influence of geography on the percentage of trials that are adequately powered.
(A) Percentage of trials with power > 0.8 for the four gender combinations of first and last author within the three country groups. Error bars represent the 95% confidence interval for proportions. (B) A logistic regression multivariable model (see "Data analysis and statistical model" below) can be used to predict the probability that a trial will have a power above a certain value. Here the predicted probabilities that trials will have power > 0.8 are plotted as a function of year for the four gender combinations in the three country groups. The predicted probabilities are averaged across medical disciplines and plotted as mean and 95% confidence intervals.
Trends over time
The percentage of adequately powered trials with a male first author and a female last author increased over time, and was higher than the percentage for other combinations in the last three decades of the study (Figure 1A, right panel). According to a logistic regression multivariable model (see "Data analysis and statistical model") the odds ratio of adequate statistical power increased each year (odds ratio: 1.03, CI: 1.02–1.03, P<0.0001; Figure 4B).
Trends across medical fields
The higher percentage of adequately powered clinical trials with a combination of a male first author and a female last author was not restricted to specific medical disciplines, although the effect sizes differed across disciplines (Figure 5). The medical fields with a relative low odds for adequate statistical power in general, as determined with the multivariable model, are: ‘complementary medicine’, ‘endocrine & metabolic’, ‘gastroenterology & hepatology’, ‘genetic disorders’, ‘health & safety at work’ and ‘heart & circulation’, all with significant odds ratios below 0.3 compared to the reference field allergy and intolerance (Table 1). The fields with most pronounced higher statistical power for male first and female last author were ‘pregnancy & childbirth’, ‘gynaecology’, ‘lungs & airways’, ‘gastroenterology & hepatology’ and ‘tobacco, drugs & alcohol’. The total number of trials for each of the four gender combinations was not equally distributed. Most trials were published by the male–male author combination (Figure 6), and this inequality in the gender of authors was found across major medical disciplines (Figure 6). Nevertheless, the number of clinical trials with a male first and last author decreased from 64.8% in the period 1975–1985 (CI 61.9–67.6) to 49.0% after 2005 (CI 48.4–49.6; Figure 7).
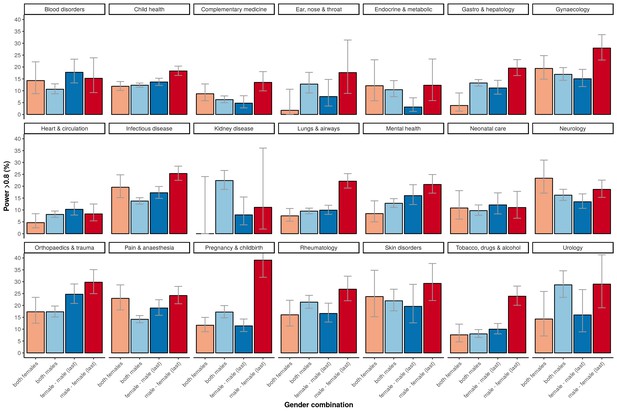
Percentage of adequately powered trials, for the four gender combinations of the first and the last author, within 21 major medical disciplines.
Error bars represent the 95% confidence interval for proportions.
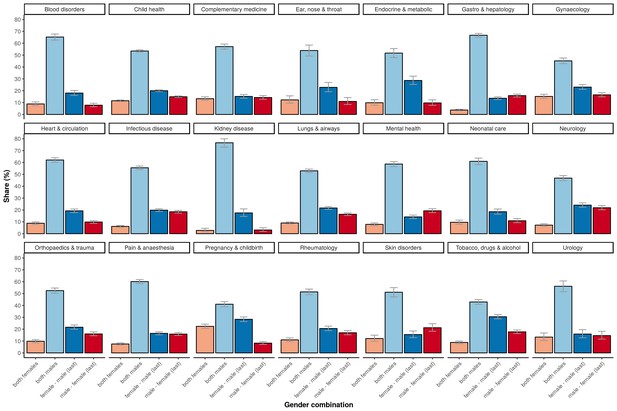
The percentage of the total number of trials underlying the four gender combinations within 21 major medical disciplines.
Error bars represent the 95% confidence interval for proportions.
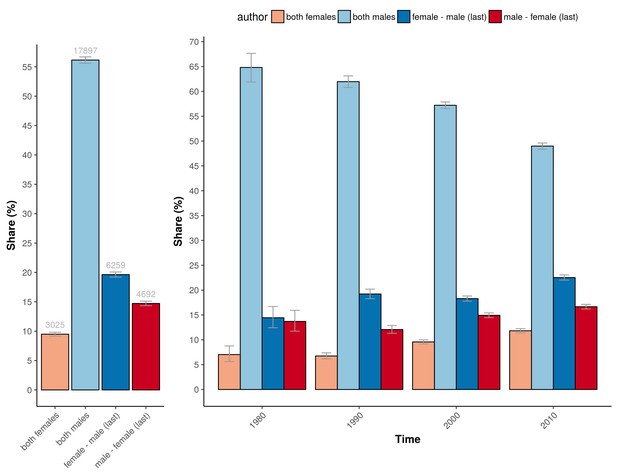
The percentage of trials for the different gender combinations and periods studied.
Left: The number and percentage of trials underlying the power calculations for the four gender combinations. Right: The corresponding percentage of the total number of trials underlying the four gender combinations for the four periods studied (1975–1985; 1985–1995; 1995–2005; >2005). Error bars represent the 95% confidence interval for proportions for both panels.
Correction for potential confounders
To correct for the potential confounders at the country level, the year of publication, and the medical discipline, logistic regression was performed. The linear combination of the variables ‘author combination’, ‘year of publication’, ‘country’ and ‘medical discipline’ explained the presence or absence of adequate statistical power well in a multivariable logistic regression model (χ2Zeng et al., 2016 = 1146.5 (degree-of-freedom 36), P<0.0001). The four author combinations were overall different from each other (χ2Zeng et al., 2016 = 440.5 (4), P<0.0001). The model estimates are provided in Table 1. A sensitivity analysis with ‘country’ defined as individual countries rather than groups of countries did not significantly change the other variable model estimates (Table 2). The sensitivity model explained the presence or absence of adequate statistical power very well in a multivariable logistic regression model (χ2Zeng et al., 2016 = 3638.6 (degree-of-freedom 101), P<0.0001). The four author combinations in the sensitivity analysis were also overall different from each other (χ2Zeng et al., 2016 = 488.2 (4), P<0.0001).
Discussion
The analysis of 31,873 clinical trials published between 1974 and 2017 demonstrates that adequately powered clinical trials are relatively more often published by a combination of a male first author and a female last author compared to other gender combinations. This effect was robust as the effect was present across countries and most medical fields. Even though the average statistical power was generally low, the overall percentage of adequately powered trials slightly increased over the past four decades.
In line with recent literature, (West et al., 2013) the absolute number of clinical trials published by female authors remained relatively low, even though it increased over time. The effects of equal representation of male and female scientists are not only important to better understand the success of collaborative efforts, but are also pressing in light of the persistent gender gap in medicine. Despite improvements, female scientists continue to face unequal pay (Rimmer, 2017) and funding disparities (Shen, 2013), and to remain underrepresented in clinical medicine in terms of the clinical faculty positions and first author publications (Jagsi et al., 2006; Reed et al., 2011), even though gains in participation have been made over the last years (Filardo et al., 2016). Independent of gender, the overall percentage of adequately powered clinical trials was disappointingly low, notwithstanding the fact that the practice of conducting clinical trials with low statistical power has been denounced for a long time (Halpern et al., 2002; Ioannidis, 2005). On a more positive note, the percentage of adequately powered trials did increase slightly over the past four decades. A possible reason for this increase may be the obligation to register clinical trials (i.e., on platforms like clinicaltrials.gov). This may have caused an increase in pre-registrations and research protocols with a higher quality and commitment to the original research plan and proposed sample size.
Our results support previous reports that gender differences exist and may influence the quality of clinical trials (Campbell et al., 2013; Nielsen et al., 2017). It may also be influenced by collaboration style patterns as differences exist between men and women in mixed-sex interactions (Balliet et al., 2011). Firm evidence on the influence of collaborative styles is still lacking. (Zeng et al., 2016; Araújo et al., 2017) However, the impact of social behavior between clinical researchers on trial outcomes – particularly related to gender - is yet a rather unexplored area. It is important to note that not all studies have found convincing evidence for gender differences in science, (Hyde, 2005) for example with regard to bias (Fanelli et al., 2017). From our results, it could be hypothesized that collaborations between male and female researchers are beneficial with respect to cross-fertilization, team productivity and research efficacy. However, our understanding of social and gender-related factors that underlie clinical trial quality is probably still limited, which is underlined by our finding that the statistical power of trials is relatively low when both first and last author are female.
Because our analyses are based on a comprehensive body of clinical trials published over a 40-year period, across medical fields, the results provide a representative picture of the relation between gender collaborations and statistical power. Nonetheless, there are several limitations. First, we only investigated one aspect of methodological rigor. Even though statistical power is an important sign of sound trial conduct, there are other domains, including pre-post registration mismatch and other sources of bias that determine methodological rigor. These parameters, however, are more difficult to quantify. Second, gender from the first and last author could not be determined for most included clinical trials (almost 70%, see flow diagram) as not all first name records were available. However, the statistical power of trials with missing gender data was not different from the clinical trials with known gender. Third, first and last authorships only provide a relative rough proxy for junior and senior positions. The actual hierarchical relations in a clinical trial may differ in a subset, for instance in some disciplinary fields authors are alphabetically positioned, or the persons in charge of the actual conduct of the clinical trial in daily practice are not last author on the resulting publication. Fourth, we only have included clinical trials and although these results can be extrapolated to other types of research, other research types and other academic disciplinary fields may have other unwritten rules how to determine the authors’ positions on a paper. Fifth, we do not have the data of the gender of the authors between the first and last author which may influence collaboration patterns within and between research groups.
Even though adequate power in clinical trials is of vital importance, (Ioannidis, 2014) future studies on gender collaborations should also take other methodological outcomes into account, such as the risk of bias and deviations from the pre-registered protocol. Also, to further determine how the gender of a researcher impacts on the scientific methodological quality, a more qualitative research design would be necessary to explore on a deeper level why methodological quality of clinical trials depends on the gender of researchers and clinicians. This would include interviews and observation studies of clinical trial teams with male and female leadership positions. Our findings demonstrate the importance of gender diversity in research collaborations and emphasize the need for more prominent positions for women at senior positions in medicine (Nature, 2013).
Materials and methods
Selection of clinical trials
Request a detailed protocolThe selection of trials for this analysis is shown in a flow chart (Figure 8). Clinical trials were extracted from the Cochrane Database of Systematic Reviews. Only the subset of trials was included in the analysis where the first name of the first and last author were reported. These reviews cover all medical fields and have high quality standards and methodological rigor with elaborate search protocols, and rigorously identify and summarize comparable trials (Jørgensen et al., 2006). Moreover, these reviews perform meta-analyses on individual clinical trials to generate an estimated effect size of interventions. All clinical trials with a PubMed ID included in a systematic review published in the second Issue of the 2017 Cochrane Database of Systematic Reviews (CDSR) were extracted using an in-house developed, open-source Cochrane Library website parser. For each individual clinical trial, we extracted publication year, outcome estimates (odds or risk ratio, risk difference or standardized mean difference), and Cochrane’s medical discipline classifications.
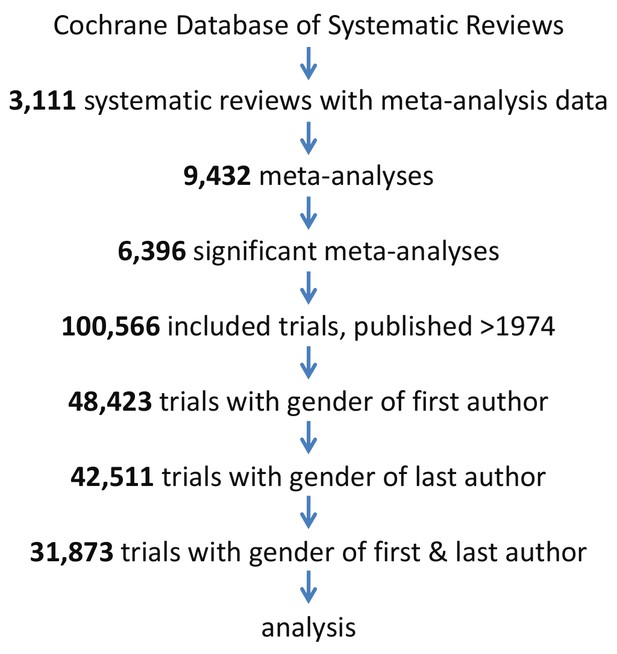
Flow diagram of the 31,873 trials selected for analysis.
Trials were analyzed if published after 1974, included in a significant meta-analysis in a systematic review and gender data was extractable for both the first and the last author.
Statistical power of individual clinical trials
Request a detailed protocolStatistical power was assessed in clinical trials, published after 1974, which were included in a Cochrane meta-analysis with a significant overall estimate (i.e., a meta-analytic P-value of <0.05). All data and scripts are available via the Open Science Framework (WM Otte, Temporal RCT power, Open Science Framework, https://osf.io/ud2jw/. Update 17-03-04 11:19 AM). We included only significant meta-analyses to exclude bias from interventions with no proven effects. In other words, if a confidence interval of a meta-analysis contains 0, the point estimate of the overall effect size is not reliable nor known and may not be used to estimate the individual power of studies included in that meta-analysis. Nevertheless, inclusion of non-significant meta-analyses did not impact on our findings (data not shown).
The power for an individual clinical trial was calculated based on the sample sizes in both trial arms, using a 5% α threshold using the meta-analytic estimate as approximation of the true effect size. Trials with a statistical power lower than 80% were considered to be underpowered based on historical arguments (Moher et al., 1994). This cut-off is standard but also relatively arbitrary. We therefore also performed analyses using a less and more conservative cut-off of 70% and 90%, respectively. The statistical power is presented in all plots with 95% confidence intervals determined with the Wilson’s score method (Wilson, 1927).
Gender extraction
Request a detailed protocolAll included trials had multiple authors. We considered the first author of clinical trial publication as a junior researcher and the last author as a senior. This assumption will most likely reflect the hierarchal relationship in the majority of the cases. The senior author having the last position in publications has long been practiced in medicine. Typically, the person conducting the practical research, analyzing the data, and drafting the first manuscript is often the first author, while the last author is the senior research responsible for the overall oversight.
For the gender of authors, first names were extracted for the first and the last author for all included clinical trials using the online interface PubReMiner (http://hgserver2.amc.nl/cgi-bin/miner/miner2.cgi) (Slater, 2014). First names were then converted to male and female probabilities with the application programming interface (API) of Genderize (http://genderize.io/). This API compares first names against a database containing over 216,000 distinct names from 79 countries and 89 languages based on millions of public profiles and their gender data in major social networks. Accuracy of female and male classification with this API, compared with open-source gender prediction tools, is excellent (Wais, 2016). A recent validation study reported female and male classification precisions of 95% and 98%, respectively (Karimi et al., 2016). Gender probabilities were dichotomized to obtain binary male/female labels. Trials with unknown gender data for either the first or last author were not included in the analysis. Missing first names caused most of the unknown genders. For some first names no gender data was available in the gender database (<5%).
Data analysis and statistical model
Request a detailed protocolClinical trials with adequate statistical power, more than 80%, were identified for all four combinations of the gender of first and last author (i.e. female–female, male–male, female–male and male–female).
To correct for potential cultural differences we determined the author’s institutional country based on the given affiliation. We only determined this for the first author as affiliations for co-authors are added to the PubMed database only since 2014. We categorized the countries into three main groups based on prevalence. The Anglosphere countries are those where English is the main native language, the European countries, except for the United Kingdom but including Ireland, were categorized in another group. The remaining countries were labeled as Non-western.
We classified the trials using the 21 standard Cochrane major medical discipline classifications. To exclude selection bias, statistical power was determined for all clinical trials with missing gender data. To ascertain that results were not due to disproportionate female underrepresentation in older trials, the absolute number of clinical trials for the four different gender combinations was also calculated.
The data were modeled with logistic regression. In this model the log odds of the dichotomous outcome variable, namely trial ‘adequate power’, was modeled as a linear combination of predictor variables. We used the glm function in R software version 3.2.0. The variable ‘author combination’ was added as a factor to the model, with the author combination ‘both females’ as reference group. The three covariates included were ‘publication year’, ‘country’ and ‘medical field’. The model fit was investigated with the significance of the overall model. This χ (Zeng et al., 2016) test determines whether the model with predictors fits significantly better than a so called null model with just an intercept. The 95% confidence intervals for the estimated coefficients were determined with the profiled log-likelihood function (Venzon and Moolgavkar, 1988).. The estimates were exponentiated to interpret them as odds-ratios. The overall effect of ‘author combination’ in the model was tested with the Wald test. We determined the model’s predicted probabilities and their 95% confidence intervals over time. We considered a P-value<0.005 as significant (Benjamin et al., 2018). We performed a sensitivity analysis with the ‘country’ variable not specified into three main categories but into individual country categories, if a minimal of fifty entries per country were present.
Data sharing
Request a detailed protocolOpen-source code to reproduce our processing pipeline is provided via the Open Science Framework (WM Otte, Temporal RCT power, Open Science Framework, https://osf.io/ud2jw/. Update 17-03-04 11:19 AM). Data extraction from the Cochrane Database of Systematic Reviews requires full text access.
Data availability
Data and scripts are available via the Open Science Framework (https://osf.io/ud2jw/) Parsing of genders can be done via genderize.io.
References
-
Sex differences in cooperation: a meta-analytic review of social dilemmasPsychological Bulletin 137:881–909.https://doi.org/10.1037/a0025354
-
Redefine statistical significanceNature Human Behaviour 2:6–10.https://doi.org/10.1038/s41562-017-0189-z
-
Power failure: why small sample size undermines the reliability of neuroscienceNature Reviews Neuroscience 14:365–376.https://doi.org/10.1038/nrn3475
-
The gender similarities hypothesisAmerican Psychologist 60:581–592.https://doi.org/10.1037/0003-066X.60.6.581
-
How to make more published research truePLoS Medicine 11:e1001747.https://doi.org/10.1371/journal.pmed.1001747
-
The "gender gap" in authorship of academic medical literature--a 35-year perspectiveNew England Journal of Medicine 355:281–287.https://doi.org/10.1056/NEJMsa053910
-
Product review: pubmed PubReMinerJournal of the Canadian Health Libraries Association 33:106–107.https://doi.org/10.5596/c2012-014
-
A method for computing profile-likelihood-based confidence intervalsApplied Statistics 37:87–94.https://doi.org/10.2307/2347496
-
Gender prediction methods based on first names with genderizeRThe R Journal 8:17–37.
-
Probable inference, the law of succession, and statistical inferenceJournal of the American Statistical Association 22:209–212.https://doi.org/10.1080/01621459.1927.10502953
Decision letter
-
Peter RodgersReviewing Editor; eLife, United Kingdom
In the interests of transparency, eLife includes the editorial decision letter and accompanying author responses. A lightly edited version of the letter sent to the authors after peer review is shown, indicating the most substantive concerns; minor comments are not usually included.
Thank you for submitting your article "Opposite sexes attract: male-female collaborations and the statistical power of 31,873 clinical trials" to eLife for consideration as a Feature Article. Your article has been reviewed by three peer reviewers, and the evaluation has been overseen by myself as the eLife Features Editor. The following individuals involved in review of your submission have agreed to reveal their identity: Andreas Neef (Reviewer #1).
The reviewers have discussed the reviews with one another and I have drafted this decision to help you prepare a revised submission
Summary:
The study presented is an analysis of over 30,000 clinical trials to assess whether gender pairings of first and last authors are associated with sufficient statistical power. The study employs a large data set and uses the API Genderize to assign gender. The authors find that overall a relatively low number of studies had sufficient statistical power (12-13%). Similar to other studies looking at gender pairings, the authors find that most studies are comprised of male first-male last authors pairings. Over the forty years analyzed, the authors observed a gradual increase in the number of female first and last authors in clinical trials. When assessed for sufficient power, studies comprised of a first male author and female last author were significantly more prevalent compared to other gender pairings.
However, a number of concerns need to be addressed before the article can be accepted for publication. In particular, there is a need for more transparency with respect to data inclusion/exclusion, and the authors need to pay more careful attention to potential confounders and possible selection bias (see points 1-8 below). The Discussion section and the references cited also need extensive attention (points 9 and 10). The Title also needs to be revised (point 11)
Essential revisions:
Confounders: I see three possible confounders that may bias the results of this study. Time, discipline-specific traditions and country variation. I will briefly touch upon each of these possible confounders below and suggest a suitable solution.
1) As mentioned by the authors, the underrepresentation of female authors in older trials may bias the results given the general increase in the percentage share of sufficiently powered trials over time. The authors account for this in Figure 1 (right panel) by dividing the results into periodical bins. But what about the possible variation within these ten-year periodical bins? A more refined approach could be to use publication year as a covariate in a binary logistic regression (I return to this).
2) Some medical disciplines may be more likely to conduct sufficiently powered trials than others, irrespective of author-gender composition. It is difficult infer whether this is the case from Figure 2. But four of the disciplines with the most pronounced higher statistical power for studies with female last authors (gynaecology, pregnancy and childbirth and tobacco and drugs and infectious diseases) also have generally good female author representation as presented in Figure 6. Consequently, the general difference detected in your study may possibly be explained by a higher general proportion of women authors in disciplines that are have relatively high general proportions of sufficiently powered studies. Or alternatively, the actual differences may possibly be larger than what you report, if the areas where women authors are well-represented are less likely to reach sufficient statistical power. Again, a more refined approach would be to do a binary logistic regression where you include covariates for the average proportion of female last authors and female authors per discipline.
3) Your study does not adjust for country-variations, which makes sense given your primary emphasis on descriptive statistics. My worry is that women's general representation as trial authors may be higher in countries that score relatively high with respect to proportion of sufficiently powered studies. If, for instance, North America (Canada and US) and Northern Europe have good numbers of women trial authors, while also being "top-performers" with respect to sufficiently powered studies, this may bias your results. Here you could also calculate a covariate adjusting for women's proportional participation as authors in a given geographic region (North America, North-Western Europe, South-East-Asia, Oceania etc.) and use this in a binary log reg.
To briefly summarize I suggest that you adjust for these possible confounders in a binary logistic regression with the outcome variable: 0=insufficiently powered 1= sufficiently powered. The regression could include your author-composition categories + the following covariates: publication year, proportion of women authors in the discipline, proportion of women last authors in the discipline, proportion of women authors in the geographical region where the trial took place, and proportion of women last authors in the geographical region where the trial took place.
4) Possible selection bias: In subsection “Statistical power of individual clinical trials” it is mentioned that you only included significant meta-analyses to exclude bias from interventions with no proven effects? I miss some sort of justification for this choice? Why would interventions with no proven effect bias your results? It would also be useful to know whether the gender composition of authors contributing to studies with no effects is similar to that of the analysed sample. Is female authors' representation higher in the sample with no proven effects relative to the sample used in the analysis?
5) Transparency with respect to data inclusion and exclusion: It would be helpful to have some sort of flow-diagram specifying the data exclusion steps. (1) How many trials did you start out with? (2) were you able to extract the necessary data from all eligible clinical trials identified in Cochrane? (3) How many studies were excluded due to statistical insignificance? (4) How many studies were excluded due to missing gender specification?
6) It would aid interpretation if the breakdown of numbers (studies excluded, numbers per category of pairing, those with sufficient statistical power….) were provided in a Table or in a Figure (similar to how presented in Figure 7).
7) Please also ensure that the following information is available in the manuscript:
- information on the definition of confidence intervals (was bootstrap used?)
- information on the statistical test applied. chi^{2}-test is mentioned, to me it is unclear if multiple comparison correction was used and why the continuity correction has to be used when actually _numbers_ of studies are tested. Also unclear is whether the bimodal distribution of statistical power in studies (see Button et al., 2013) would still have us expect a normal distribution (or hypergeometric) of the number of cases in each class. Even more concerning is the potential internal structure in the dataset: how many different seniors carry the effect? etc.
So to address all this within one analysis: Why not use randomized gender label swapping?
8) A more elaborate description of the gender disambiguation would also strengthen the study. In subsection “Gender extraction” you mention that "gender probabilities were dichotomized to obtain binary male/female labels". I presume this means that your gender algorithm provides numerical name-to-gender accuracy estimations? (please describe in more detail how the algorithm works). If yes, what threshold did you use? It would also strengthen the study if you could manually check a sub-sample of authors to verify the accuracy of the algorithm with the chosen threshold.
9) A number of reference (e.g., Helmer et al., 2017; Wuchty, Jones and Uzzi, 2007; Jones, Wuchty and Uzzi, 2008; Woolley et al., 2010 and Rhoten and Pfirman, 2007) are quoted out of context. Woolley et al., 2010 does not focus on research teams: rather, it finds that the collective intelligence of teams increase with women's representation, not with gender diversity. Rhoten and Pfirman, 2007 finds that women (not gender diverse teams) are more likely to do interdisciplinary research.
10) The Discussion section needs to be completely rewritten to focus on:i) how the results in the present manuscript relate to the existing literature (including possible explanations for the results).ii) the shortcomings and caveats associated with the present approach (e.g., just one type of article, just one area of science, just the first and last authors); and only first/last author). iii) open questions for future research.
As mentioned above, please ensure that any papers cited are directly relevant to the subject being discussed.
11) The Title needs to be revised to better reflect the content of the article. Also, please note that punctuation like colons, semi-colons and hypens/dashes are not allowed in the title of eLife articles.
[Editors' note: further revisions were requested prior to acceptance, as described below.]
Thank you for submitting a revised version of your manuscript "Adequate statistical power of clinical trials is associated with the combination of a male first author and a female last author". The revised version has been reviewed again by one of the referees who reviewed the original version. Once you have satisfactorily addressed the comments from this reviewer (see below) and some editorial comments from myself (also below), we should be able to accept your article.
The authors have done a good job addressing my main concerns about confounders. The logistic regression analysis has strengthened the study a lot. I think the paper is ready for publication with some revisions.
1) The authors used categorical values with three values to adjust for geo-cultural variation. I am not convinced that this is sufficient, and given the size of the data-set, I suggest that they run the same log-reg model with dummy for all countries to ensure that potential confounders at the country level are sufficiently adjusted for. If this model lead to the same results, they can choose to report the model as it is given now.
2) I suggest that the authors revise the Results section so that descriptive findings are presented first, followed by the outcomes of the logistic regression analysis. Mixing the results from the different approaches is somewhat confusing. The log-reg should be used to validate the descriptive results.
3) Regarding the Discussion section: I am still not convinced by the arguments about gender differences in collaboration style patterns. It is difficult for me to understand how your research adds to discussions of collaboration style. Collaboration style is a question of process, your study can only contribute to the understanding of outcomes? I would argue that your study contributes to the emerging literature about how gender diversity may influence research outcomes. Here are some references for other relevant studies addressing this issue (one of which – Campbell et al., 2013 – is already cited):
# Valantine and Collins (2015) (This paper could work quite well as a motivation for your manuscript])
# Nielsen et al., (2017)
# Nielsen et al., (2017)
# Joshi, (2014)
# Campbell et al., (2013)
4) Likewise, I am not convinced by the speculative argument that pairs of female first and last authors have less powered studies due to women seniors being more critical of female employees. How would being critical towards female coauthors lower statistical power? I can't see the link, and I suggest that you skip this reflection entirely.
5) Woolley et al., 2010 is not about research teams, but teams in general.
6) I feel the Title would read better if the fourth word was changed from "of" to "in" so that the title read as follows:
Adequate statistical power in clinical trials is associated with the combination of a male first author and a female last author.
However, please feel free to keep the present title if you wish.
7) I feel that the abstract and introduction would read better as follows:
Abstract
“Clinical trials have a vital role in ensuring the safety and efficacy of new medical treatments and interventions. A key characteristic of a clinical trial is its statistical power. Here we investigate whether the statistical power of a trial is related to the gender of first and last authors on the paper reporting the results of the trial. Based on an analysis of 31,873 clinical trials published between 1974 and 2017, we find that adequate statistical power was most often present in clinical trials with a male first author and a female last author (20.6%, 95% confidence interval 19.4-21.8%), and that the difference between this figure and the figure for other gender combinations was significant (12.5-13.5%; P < 0.0001). The absolute number of female authors in clinical trials also increased gradually over time, with the percentage of female last authors rising from 20.7% (1975-85) to 28.5% (after 2005). Our results demonstrate the importance of gender diversity in research collaborations and emphasize the need to increase the number of women in senior positions in medicine.”
Introduction section
“Clinical trials are complex projects that often involve collaborations between researchers who have different areas of expertise and different levels of seniority. The statistical power of a clinical trial reflects the chance of detecting a true effect, so adequate statistical power is regarded as one of the key elements of responsible research [8] and is considered essential in reproducible clinical research [9]. However, there is an increasing awareness that many clinical trials have systematic methodological flaws, including a lack of adequate statistical power, and that their results may be biased, exaggerated, and difficult to reproduce [1].
Male and female researchers differ in their collaborative strategies in ways that depend on their levels of expertise and whether they have a junior or senior position [4,5]. There are also indications that mixed gender research groups may make the best use of personal knowledge and skills [6,7], but possible relationships between the gender balance of collaborations and the quality of clinical trials – as measured by their statistical power – have not been investigated. In this study, we examined 31,873 clinical trials published between 1974 and 2017 to see if there was any relation between the gender of the first and last authors and statistical power. We found that the probability of having adequate statistical power for one combination – male first author, female last author – was significantly higher than that for the other three possible combinations. Moreover, this effect was present across countries and most medical fields.”
Please feel free to revise the Abstract and Introduction section further, but please try to avoid undue overlap between the two. Also, please note that my suggestions would mean that the following sentence (and the references therein) would be deleted: "Indeed, collaborations on trial design, conduct and report are important, and the publications resulting from team efforts and multi-university research teams are more often cited and have more impact [2,3]."
8) Textual changes and clarifications:
# Please list all the Anglophone in parenthesis the first time the word Anglophone is used.
# Please clarify in the UK and Ireland are included with the Anglophone or European countries.
# Please list the five biggest non-Western countries in parenthesis the first time the phrase non-Western countries is used.
# In the third paragraph of the Discussion, please reword the passage "It is important to note that gender assumptions are not black and white" to be more precise.
# In the fourth paragraph of the Discussion section, please replace the first two sentences ("Our analyses […] statistical power") with one shorter sentence.
9) Figures
To help the presentation of the article, please do the following:
# combine Figure 1, Figure 2 and Figure 3 into one figure with three panels.
# combine Figure 6 and Figure 7 into one figure with two panels.
# please add a colour scale to the present Figure 5.
# please expand the caption for the present Figure 7 to better explain what has been measured and what is being predicted.
https://doi.org/10.7554/eLife.34412.015Author response
Summary:
The study presented is an analysis of over 30,000 clinical trials to assess whether gender pairings of first and last authors are associated with sufficient statistical power. The study employs a large data set and uses the API Genderize to assign gender. The authors find that overall a relatively low number of studies had sufficient statistical power (12-13%). Similar to other studies looking at gender pairings, the authors find that most studies are comprised of male first-male last authors pairings. Over the forty years analyzed, the authors observed a gradual increase in the number of female first and last authors in clinical trials. When assessed for sufficient power, studies comprised of a first male author and female last author were significantly more prevalent compared to other gender pairings.
However, a number of concerns need to be addressed before the article can be accepted for publication. In particular, there is a need for more transparency with respect to data inclusion/exclusion, and the authors need to pay more careful attention to potential confounders and possible selection bias (see points 1-8 below). The Discussion section and the references cited also need extensive attention (points 9 and 10). The Title also needs to be revised (point 11).
We thank the editor and all reviewers for their thoughtful and constructive feedback. Our feedback is provided below.
Essential revisions:
[…] To briefly summarize I suggest that you adjust for these possible confounders in a binary logistic regression with the outcome variable: 0=insufficiently powered 1= sufficiently powered. The regression could include your author-composition categories + the following covariates: publication year, proportion of women authors in the discipline, proportion of women last authors in the discipline, proportion of women authors in the geographical region where the trial took place, and proportion of women last authors in the geographical region where the trial took place.
We agree with the reviewers that the factors ‘study year’, ‘country of author’ and ‘medical discipline’ may affect the association between ‘author type’ and the dependent variable ‘sufficiently powered’. For instance, as seen in Figure 1, there appears to be a change in adequately powered trials over time.
We re-analyzed the data with logistic regression (with outcome variable: 0=insufficiently powered 1= sufficiently powered) where ‘study year’, ‘country of author’ and ‘medical discipline’ were included as a covariates. We have added Table 1 with the corresponding odds ratio’s, 95% confidence intervals and P-values to the revised manuscript. We have also added predicted probability plots, with the 95% prediction intervals to help understand the model.
4) Possible selection bias: In subsection “Statistical power of individual clinical trials” it is mentioned that you only included significant meta-analyses to exclude bias from interventions with no proven effects? I miss some sort of justification for this choice? Why would interventions with no proven effect bias your results? It would also be useful to know whether the gender composition of authors contributing to studies with no effects is similar to that of the analysed sample. Is female authors' representation higher in the sample with no proven effects relative to the sample used in the analysis?
In the frequentist framework we cannot infer information from the point estimate of a meta-analysis if the confidence interval contains 0. As we do not know the true effect size (which could also be 0) we can also not reliable estimate the statistical power, as this requires an estimation of the true effect size. Even so, inclusion of non-significant meta-analyses did not change any of the results. We have added text and clarification in the article.
We have added data from the trials where we have no gender information. The average power for those studies is very similar to the categories ‘both females/both males/female-male (last)’, indicating that our data is not biased by these ‘unknowns’ (see Figure 7).
5) Transparency with respect to data inclusion and exclusion: It would be helpful to have some sort of flow-diagram specifying the data exclusion steps. (1) How many trials did you start out with? (2) were you able to extract the necessary data from all eligible clinical trials identified in Cochrane? (3) How many studies were excluded due to statistical insignificance? (4) How many studies were excluded due to missing gender specification?
We have constructed a flow diagram (Figure 1) and additional descriptive figures in the revised manuscript. Furthermore, we explain briefly why our gender identification software was unable to detect gender in almost 70% of the included trials (Discussion section).
6) It would aid interpretation if the breakdown of numbers (studies excluded, numbers per category of pairing, those with sufficient statistical power….) were provided in a Table or in a Figure (similar to how presented in Figure 6).
These numbers are now given in the flow diagram (Figure 8).
7) Please also ensure that the following information is available in the manuscript:
- information on the definition of confidence intervals (was bootstrap used?)
All confidence intervals are 95% confidence intervals.
We have added this to the manuscript. All confidence intervals are 95% confidence intervals. The CI’s for the proportions are determined with the standard R function prop.test from the default ‘stats’ package (https://stat.ethz.ch/R-manual/R-devel/library/stats/html/prop.test.html). The confidence intervals for the odds ratios from the logistic regression model are determined with the confint function in R from the ‘stats’ package (http://stat.ethz.ch/R-manual/R-devel/library/stats/html/confint.html).
- information on the statistical test applied. chi^{2}-test is mentioned, to me it is unclear if multiple comparison correction was used and why the continuity correction has to be used when actually _numbers_ of studies are tested. Also unclear is whether the bimodal distribution of statistical power in studies (see Button et al., 2013) would still have us expect a normal distribution (or hypergeometric) of the number of cases in each class. Even more concerning is the potential internal structure in the dataset: how many different seniors carry the effect? etc.
So, to address all this within one analysis: Why not use randomized gender label swapping?
As we changed the statistical testing to logistic regression the statistical analysis with chi-square is removed. We only fitted one model in our current manuscript version. We have lowered the α cutoff threshold to 0.005, based on a recent discussion on the too liberal threshold of 0.05 (Benjamin et al., 2018).
8) A more elaborate description of the gender disambiguation would also strengthen the study. In subsection “Gender extraction” you mention that "gender probabilities were dichotomized to obtain binary male/female labels". I presume this means that your gender algorithm provides numerical name-to-gender accuracy estimations? (please describe in more detail how the algorithm works). If yes, what threshold did you use? It would also strengthen the study if you could manually check a sub-sample of authors to verify the accuracy of the algorithm with the chosen threshold.
The gender extraction based on first names with genderize.io has the highest accuracy of current methods available at the moment. Comparison and validation work is reported in this paper (Wais, (2016)). The genderize.io method is compared to the method of West et al., (2013) and Larivière et al., (2013). The area-under-the ROC curve – a general measure of predictiveness – for the genderize.io software is 0.927. The predicted power is thus excellent. Another study which validated the high validity of generize.io gender prediction is the work by Fell and König, (2016). Yet another study, by Topaz et al., on gender predictions in mathematical sciences, report an accuracy of 97.5% of generize.io (Topaz and Sen, (2016)) The generize.io software provides a probability for a first name being male or female (summing to 1.0). We labeled first names based on the highest gender probability.
9) A number of references (e.g., Helmer et al., 2017; Wuchty, Jones and Uzzi, 2007; Jones, Wuchty and Uzzi, 2008; Woolley et al., 2010 and Rhoten and Pfirman, 2007) are quoted out of context. Woolley et al., 2010 does not focus on research teams: rather, it finds that the collective intelligence of teams increase with women's representation, not with gender diversity. Rhoten and Pfirman, 2007 finds that women (not gender diverse teams) are more likely to do interdisciplinary research.
We appreciate the feedback. We have adjusted the references and text to fit the context better. As for West, Jacquet and King, 2013, we think the referee was actually discussing another paper (Araujo, Araujo and Moreira, 2017). This article does show that when an article has more collaborators, the chance of having interdisciplinary collaboration is higher for female scientists. We believe that our use of this reference is justified, stating that “our results do support previous findings that gender differences exist in collaboration style patterns.”
10) The Discussion section needs to be completely rewritten to focus on:i) how the results in the present manuscript relate to the existing literature (including possible explanations for the results).ii) the shortcomings and caveats associated with the present approach (eg, just one type of article, just one area of science, just the first and last authors); and only first/last author). iii) open questions for future research.
As mentioned above, please ensure that any papers cited are directly relevant to the subject being discussed.
We have rewritten the Discussion section, addressed the abovementioned shortcomings and put a focus on future research.
11) The Title needs to be revised to better reflect the content of the article. Also, please note that punctuation like colons, semi-colons and hypens/dashes are not allowed in the title of eLife articles.
We have changed the title to “Adequate statistical power of clinical trials is associated with the combination of a male first author and a female last author.”
[Editors' note: further revisions were requested prior to acceptance, as described below.]
The authors have done a good job addressing my main concerns about confounders. The logistic regression analysis has strengthened the study a lot. I think the paper is ready for publication with some revisions.
1) The authors used categorical values with three values to adjust for geo-cultural variation. I am not convinced that this is sufficient, and given the size of the data-set, I suggest that they run the same log-reg model with dummy for all countries to ensure that potential confounders at the country level are sufficiently adjusted for. If this model lead to the same results, they can choose to report the model as it is given now.
Table 2 is added to the manuscript with individual countries as potential confounder. The main effect remains and is not influenced by individual countries. We have also addressed this in the Results section.
2) I suggest that the authors revise the Results section so that descriptive findings are presented first, followed by the outcomes of the logistic regression analysis. Mixing the results from the different approaches is somewhat confusing. The log-reg should be used to validate the descriptive results.
This is a valuable suggestion. We have changed the Results section; the section “model performance” was renamed “correction for potential confounders” and moved to the end of the Results section.
3) Regarding the Discussion section: I am still not convinced by the arguments about gender differences in collaboration style patterns. It is difficult for me to understand how your research adds to discussions of collaboration style. Collaboration style is a question of process, your study can only contribute to the understanding of outcomes? I would argue that your study contributes to the emerging literature about how gender diversity may influence research outcomes. Here are some references for other relevant studies addressing this issue (one of which – Campbell et al., 2013 – is already cited):
# Valantine and Collins, (2015) (This paper could work quite well as a motivation for your manuscript)
# Nielsen et al., (2017)
# Nielsen et al., (2017)
# Joshi, (2014)
# Campbell et al., (2013)
We have added your suggested references to further improve the manuscript by adding text to the discussion. Now we state that the results suggest that gender differences may alter research outcomes. See the Discussion section.
4) Likewise, I am not convinced by the speculative argument that pairs of female first and last authors have less powered studies due to women seniors being more critical of female employees. How would being critical towards female coauthors lower statistical power? I can't see the link, and I suggest that you skip this reflection entirely.
We have removed the speculative arguments (Discussion section).
5) Woolley et al., 2010 is not about research teams, but teams in general.
We changed the sentence in the Introduction section to: “There are indications that mixed gender teams may make the best use of personal knowledge and skills, an effect also reported in a scientific research context.”
6) I feel the Title would read better if the fourth word was changed from "of" to "in" so that the title read as follows:
Adequate statistical power in clinical trials is associated with the combination of a male first author and a female last author.
However, please feel free to keep the present title if you wish.
Thank you for the suggestion, we changed the Title accordingly.
7) I feel that the abstract and introduction would read better as follows:
Abstract
“Clinical trials have a vital role in ensuring the safety and efficacy of new medical treatments and interventions. A key characteristic of a clinical trial is its statistical power. Here we investigate whether the statistical power of a trial is related to the gender of first and last authors on the paper reporting the results of the trial. Based on an analysis of 31,873 clinical trials published between 1974 and 2017, we find that adequate statistical power was most often present in clinical trials with a male first author and a female last author (20.6%, 95% confidence interval 19.4-21.8%), and that the difference between this figure and the figure for other gender combinations was significant (12.5-13.5%; P < 0.0001). The absolute number of female authors in clinical trials also increased gradually over time, with the percentage of female last authors rising from 20.7% (1975-85) to 28.5% (after 2005). Our results demonstrate the importance of gender diversity in research collaborations and emphasize the need to increase the number of women in senior positions in medicine.”
Introduction section
“Clinical trials are complex projects that often involve collaborations between researchers who have different areas of expertise and different levels of seniority. The statistical power of a clinical trial reflects the chance of detecting a true effect, so adequate statistical power is regarded as one of the key elements of responsible research [8] and is considered essential in reproducible clinical research [9]. However, there is an increasing awareness that many clinical trials have systematic methodological flaws, including a lack of adequate statistical power, and that their results may be biased, exaggerated, and difficult to reproduce [1].
Male and female researchers differ in their collaborative strategies in ways that depend on their levels of expertise and whether they have a junior or senior position [4,5]. There are also indications that mixed gender research groups may make the best use of personal knowledge and skills [6,7], but possible relationships between the gender balance of collaborations and the quality of clinical trials – as measured by their statistical power – have not been investigated. In this study, we examined 31,873 clinical trials published between 1974 and 2017 to see if there was any relation between the gender of the first and last authors and statistical power. We found that the probability of having adequate statistical power for one combination – male first author, female last author – was significantly higher than that for the other three possible combinations. Moreover, this effect was present across countries and most medical fields.”
Please feel free to revise the Abstract and Introduction section further, but please try to avoid undue overlap between the two. Also, please note that my suggestions would mean that the following sentence (and the references therein) would be deleted: "Indeed, collaborations on trial design, conduct and report are important, and the publications resulting from team efforts and multi-university research teams are more often cited and have more impact [2,3]."
Many thanks for improving the readability of the text. The Abstract is completely incorporated, and we have added some results in the Abstract. The Introduction section was also amended. We have added one line to the Acknowledgments section in appreciation of your and the reviewers’ suggestions.
8) Textual changes and clarifications:
# Please list all the Anglophone in parenthesis the first time the word Anglophone is used.
We removed the mixed of anglophone and anglosphere from the text and kept anglosphere. These are formally defined as the United States, Canada, Australia, New Zealand and the United Kingdom. We have added those the first time the word Anglosphere is used.
# Please clarify in the UK and Ireland are included with the Anglophone or European countries.
Ireland is analyzed as being part of the European category. We have added this to the text.
# Please list the five biggest non-Western countries in parenthesis the first time the phrase non-Western countries is used.
We have added the five biggest non-Western countries: Turkey, Japan, India, China, and Israel.
# In the third paragraph of the Discussion section, please reword the passage "It is important to note that gender assumptions are not black and white" to be more precise.
# In the fourth paragraph of the Discussion section, please replace the first two sentences ("Our analyses […] statistical power") with one shorter sentence.
The abovementioned changes have been made.
9) Figures
To help the presentation of the article, please do the following:
# combine Figure 1, Figure 2 and Figure 3 into one figure with three panels.
# combine Figure 6 and Figure 7 into one figure with two panels.
# please add a colour scale to the present Figure 5.
# please expand the caption for the present Figure 7 to better explain what has been measured and what is being predicted.
The abovementioned changes have been made.
https://doi.org/10.7554/eLife.34412.016Article and author information
Author details
Funding
ZonMw (445001002)
- Willem M Otte
- Joeri K Tijdink
- Herm J Lamberink
- Christiaan H Vinkers
VENI (016.168.038)
- Willem M Otte
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Acknowledgements
We appreciate the valuable input and suggestions by the reviewers. We gratefully acknowledge the possibility that the all-male author list may have negatively affected the scientific quality of our work.
Publication history
- Received:
- Accepted:
- Version of Record published:
Copyright
© 2018, Otte et al.
This article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use and redistribution provided that the original author and source are credited.
Metrics
-
- 6,728
- views
-
- 244
- downloads
-
- 8
- citations
Views, downloads and citations are aggregated across all versions of this paper published by eLife.
Citations by DOI
-
- 8
- citations for umbrella DOI https://doi.org/10.7554/eLife.34412
Download links
Downloads (link to download the article as PDF)
Open citations (links to open the citations from this article in various online reference manager services)
Cite this article (links to download the citations from this article in formats compatible with various reference manager tools)
Further reading
-
The study of science itself is a growing field of research.







