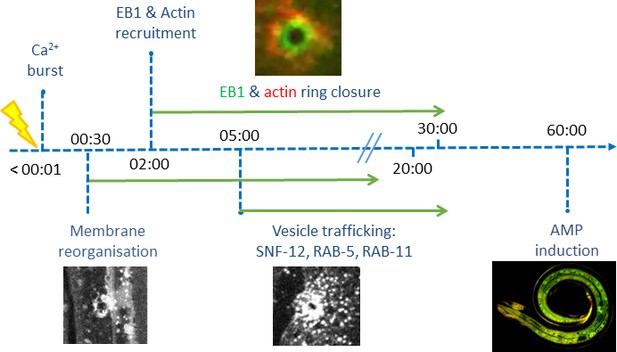
Microtubule plus-end dynamics link wound repair to the innate immune response
Figures
Using a 405 nm laser to wound the worm epidermis reveals rapid membrane reorganisation.
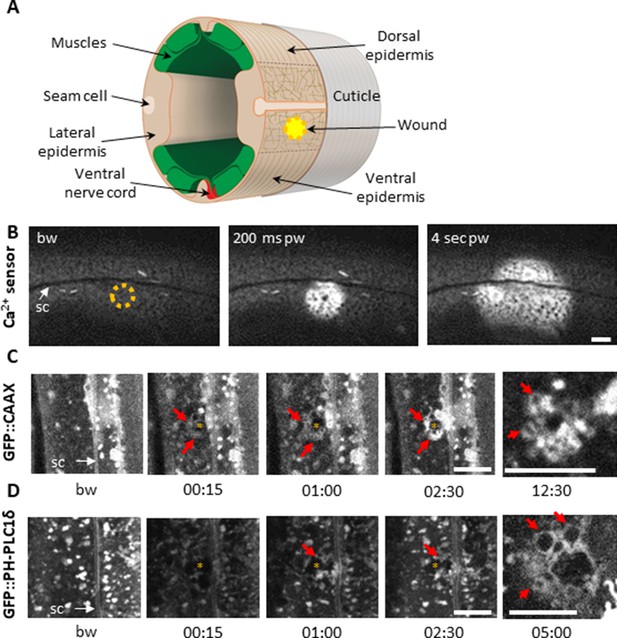
(A) Schematic view of a section through an adult C. elegans worm near the mid-body. The internal organs are omitted for the sake of clarity. The main syncytial epidermis, hyp7 (buff), with its adjacent cells (seam cells in pink, nerve cord in red and muscles in green) can be divided into two main regions, the lateral epidermis (delimited by the dashed black line) and the thin dorso-ventral epidermis where muscles are anchored to the cuticle. Microtubules (thin taupe lines) are disordered on the apical surface of the lateral epidermis. The typical position of a wound is indicated by the yellow circle. Figure adapted from one kindly provided by Christopher Crocker, WormAtlas (Altun and Hall, 2014). (B) Wounding the lateral epidermis with a 405 nm laser causes a rapid increase in intracellular Ca2+, measured using GCamp3. Representative spinning disk images from a worm carrying a col-19p::GCamp3 reporter transgene. The dashed circle is centred on the wound site; bw, before wound; pw, post-wound; sc, seam cells. Scale bar 10 µm. (C–D) Upon laser wounding, membrane and lipids (red arrows) are rapidly recruited to the wound site (asterisk). Representative spinning disk images of worms carrying dpy-7p::GFP::CAAX (C) and wrt-2p::GFP::PH-PLC1δ transgenes (D). bw, before wound; time post-wound [min:s]; sc, seam cells. Scale bar 5 µm. The last panel on the right in both C and D is a focal plane two microns deeper than the previous images, showing recruitment of cytoplasmic vesicles to the wound.
Reproduction of known wound hallmarks following injury using a 405 nm laser.
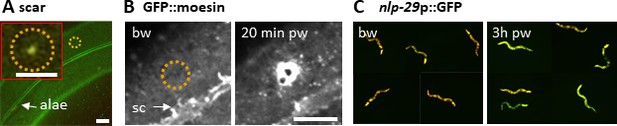
Wounding C. elegans lateral epidermis with a 405 nm laser causes immediate formation of an autofluorescent scar (A) and later an actin ring, revealed with the actin-binding protein GFP::moesin (B) at the wound site. Representative images of wild-type (A) and col-19p::GFP::moesin (B) worms. The dashed circle is centred on the wound site; bw, before wound; pw, post wound; sc, seam cells. Scale bar 10 µm except in the inset 5 µm. (C) 3 hr after wounding, young adult worms exhibit an increased expression of a nlp-29p::GFP reporter, a read out of the immune response (right), compared to unwounded worms (left). The worms express DsRed constitutively in the epidermis. Red and green fluorescence are visualized here simultaneously.
Plasma membrane rapidly reorganizes at the wound site.
Imaging Ex dpy-7p::GFP::CAAX; * indicates the wound site; [min:sec]. Scale bar 5 µm.
Rapid PIP2 domain reorganization at the wound site.
Imaging Is wrt-2p::GFP::PH-PLC1δ; * indicates the wound site; [min:sec]. At resting conditions, PIP2 membrane domains are large and static. Upon wounding, the GFP signal first disappears in a large area around the wound (15 s), it then re-appears and the domains close to the wound reorganise. Scale bar 5 µm.
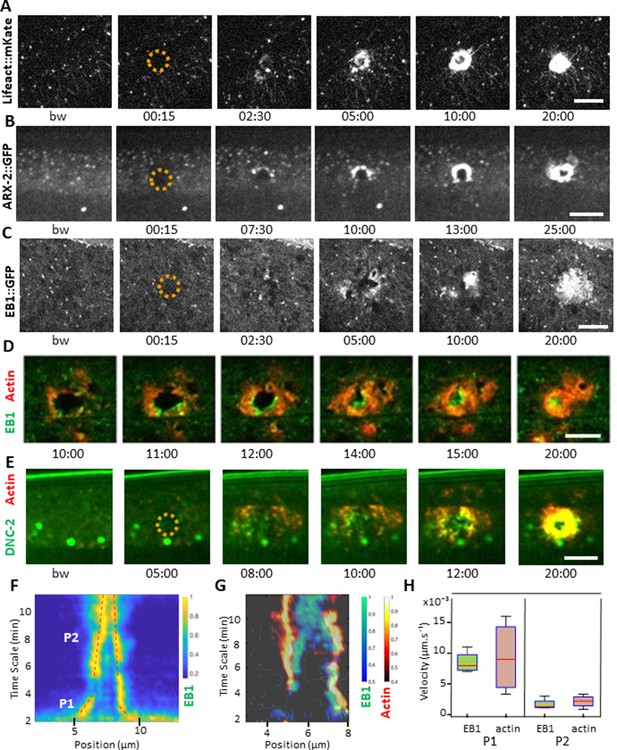
EB1 associates with actin at the wound site in the lateral epidermis.
(A) In unwounded worms, in the lateral epidermis actin is sparsely structured. Laser wounding causes actin recruitment (as early as 2.5 min) and actin ring formation (clearly visible by 5 min). These rings close with time. Representative spinning disk images of a strain carrying a col-62p::Lifeact::mKate reporter. Laser wounding also causes ARP2/ARX-2 and EB1 recruitment at the wound site as visualized in strains ARX-2::GFP KI (B) and col-19p::EBP-2::GFP (C), respectively. Simultaneous visualization of MTs (green) and actin (red) using strains carrying EB1::GFP (D) or DNC-2::GFP (E) together with col-62p::Lifeact::mKate2. The dashed circle is centred on the wound site; bw, before wound; time post-wound [min:s]; scale bar 10 µm. (F) Kymograph of EB1 signal along the anterio-posterior (AP) direction normalised according to maximal intensity, see Materials and methods, reveals two phases of fast (P1) and slow (P2) contraction. (G) Kymograph of EB1 (green) and actin (red) during wound closure, EB1 ring contraction precedes that of actin. (H) Velocities of contraction in the fast (P1) and slow phases for EB1 and actin (n = 4).
-
Figure 2—source data 1
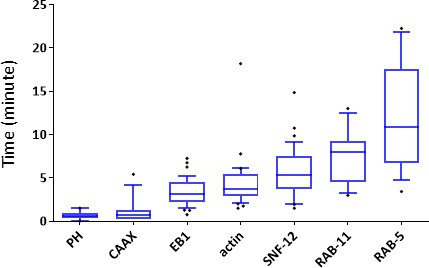
Reporter protein dynamics in the epidermis.
- https://cdn.elifesciences.org/articles/45047/elife-45047-fig2-data1-v2.docx
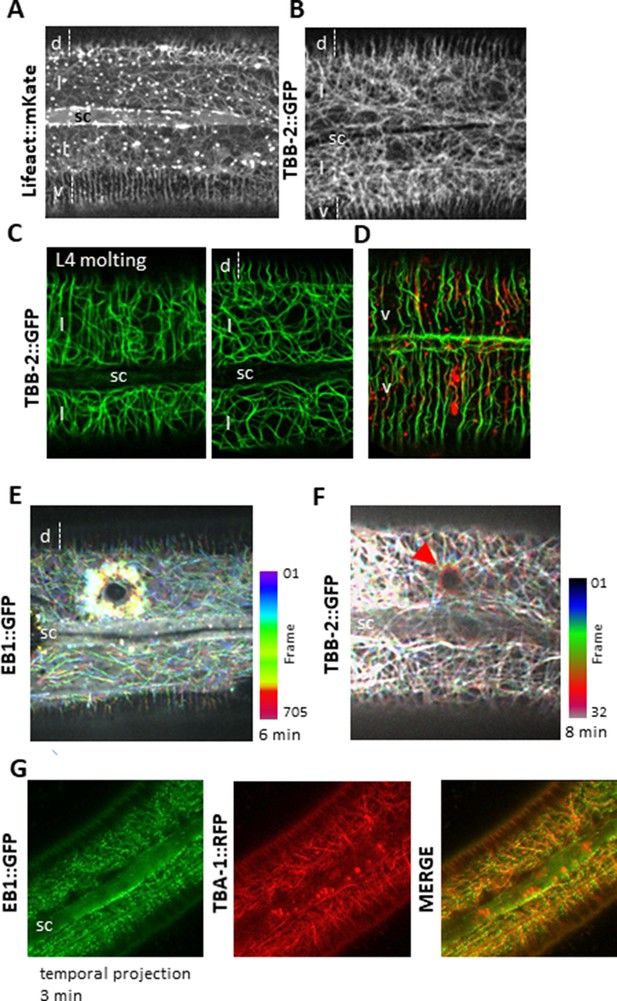
Cytoskeleton organisation and dynamic in the lateral epidermis.
Actin and microtubules are randomly organised in the lateral epidermis (l) compare to ventral (v) and dorsal (d) regions over the muscle quadrants. Representative spinning disk images of young adult worms carrying Lifeact::mKate (A) or TBB-2::GFP KI reporter. (C) During the L4-adult molt (left), MTs in the lateral epidermis are circumferentially organised, in contrast to those in the young adult (right). (D) In the ventral epidermis in a young adult, MT and actin are circumferentially organised. Representative confocal images of worms carrying TBB-2::GFP KI (C) or TBB-2::GFP KI and Lifeact::mKate (D) reporters. After wounding, EB1 (E) and MTs (F, highlighted with a red arrow) reorganise around the wound as represented with a colour coded temporal projection (0.2 s or 15 s frame interval, respectively). (G) Temporal projection of spinning disk images acquired over 3 min of worms carrying EB1::GFP and TBA-1::RFP demonstrating that EB1 comets (green) run along MTs (red); sc, seam cell, scale bar 10 µm.
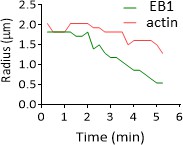
EB1 precedes actin during wound closure.
Radial profile where the radius between the maximum intensity is represented over time for EB1 in green and actin in red (strain carrying both col-62p::Lifeact::mKate and col-19p::EBP-2::GFP reporter).
Actin reorganization at the wound site.
Imaging Si col-62p::Lifeact::mKate2 strain; * indicates the wound site; [min:sec]. Scale bar 5 µm.
ARP2/3 complex is recruited at the wound site.
Imaging KI ARX-2::GFP strain; * indicates the wound site; [min:sec]. Scale bar 5 µm.
EB1/EBP-2, a plus end MT binding protein, is recruited at the wound site.
This movie represents a different wound from the one in Figure 2C. Imaging Ex col-19p::EBP-2::GFP strain; * indicates the wound site; [min:sec]. Scale bar 5 µm.
EB1 and actin are recruited at the wound site.
Figure 2—videos 4 and 5 represent two different wounds, Figure 2—video 4 being the merge of Figure 2—videos 1 and 3. Imaging Si col-62p::Lifeact::mKate2; Ex col-19p::EBP-2::GFP strain; * indicates the wound site; [min:sec]. Scale bar 5 µm.
EB1 and actin are recruited at the wound site.
Kymograph analyses of EB1 and actin at the wound site.
Imaging Si col-62p::Lifeact::mKate2, Ex col-19p::EBP-2::GFP strain (confocal) (left) and a kymograph (right) of the wound in the anterio-posterior (AP) direction normalised according to the maximal intensity of both signals. The progression of time in the movie is synchronised to the movement of the white horizontal bar moving along the kymograph.
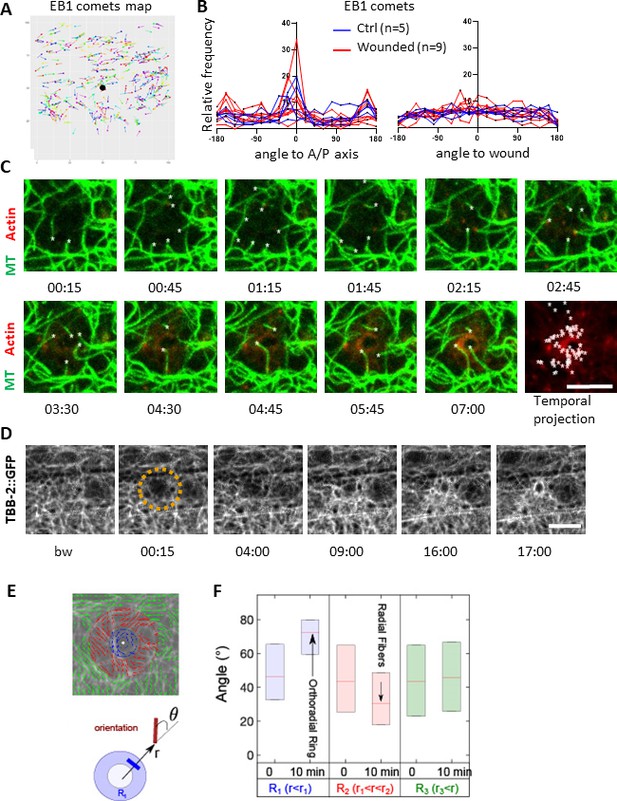
Microtubules are dynamic and orthoradially organised at the wound site.
(A) EB1 comets are tracked and mapped in a region of 150 × 150 pixels centred on the wound (black dot) for 5 min. (B) The angles of the EB1 comet vectors are calculated either to the A/P axis of the worm (left) or to the wound or the centre of a control region (right); their relative frequency are compared between control (n = 5) and wounded regions (n = 9). (C) Representative confocal images of a strain carrying a col-62p::Lifeact::mKate2 and TBB-2::GFP reporter; time post-wound [min:sec]; scale bar 5 µm. An asterisk marks the position of the tips of MTs extending into the site of the wound. The final panel is a temporal projection (Figure 3—video 1). (D) Representative spinning disk images of a strain carrying a TBB-2::GFP reporter. The dashed circle is centred on the wound site; time post-wound [min:sec]; scale bar 5 µm. (E) Map of the orientation of MTs 10 min post wounding, coloured according to their distance to the wound centre, defining sectors R1 (blue), R2 (red), R3 (green) in a MAPH-1::GFP reporter strain. Definition of the angle θ between the local direction of MT and the direction to the wound centre. (F) Statistics of the orientation θ for each sector just before (t = 0 min) and after (10 min) wounding. The increase in the mean value of θ in R1 corresponds to orthoradial MTs; the decrease in angle in R2 corresponds to the appearance of radially oriented structures; no global structures were observed at longer distances R3 (n = 3).
Microtubules regrow around the wound site in front of actin.
Imaging KI TBB-2::GFP strain; Si col-62p::Lifeact::mKate2 (confocal); * indicates the wound site; [min:sec]. Scale bar 5 µm.
Microtubules knit a web around the wound site.
Imaging KI TBB-2::GFP strain; * indicates the wound site; [min:s]. Scale bar 5 μm.
Imaging KI MAPH-1::GFP strain; * indicates the wound site; [min:s].
Scale bar 5 μm.
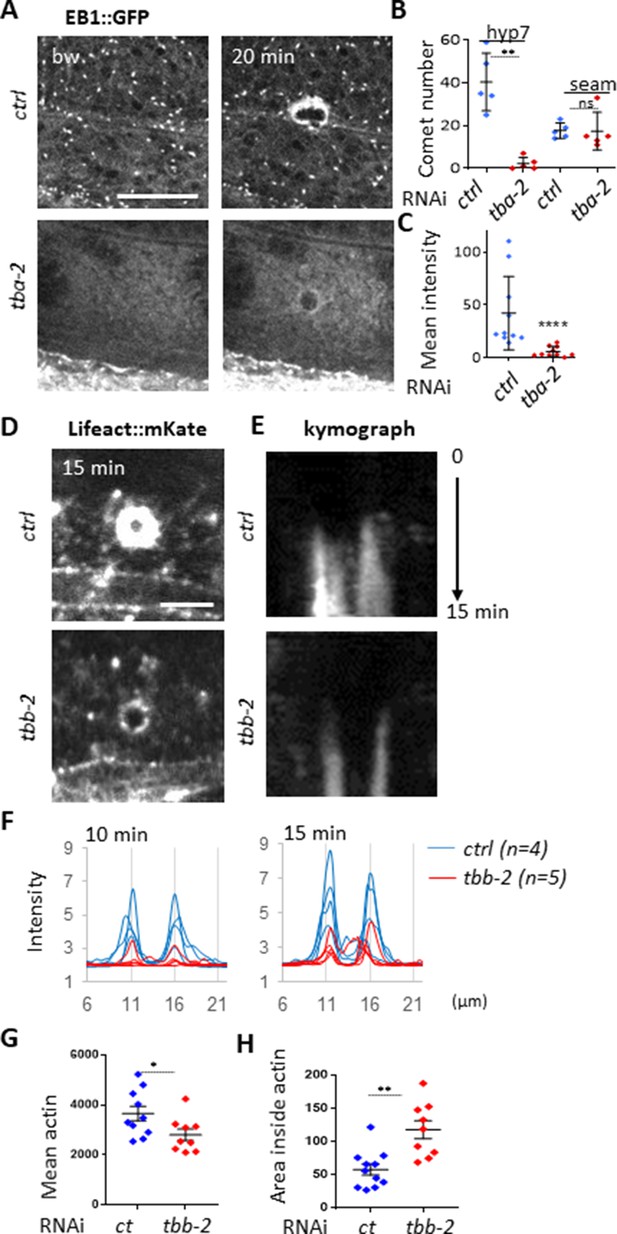
Non-centrosomal microtubule dynamics are required for actin ring closure upon wounding.
(A) Tubulin α TBA-2 is required for the presence of EB1 comets and EB1 recruitment at the wound site. Representative spinning disk images of col-19p::EBP-2::GFP in control (ctrl) and tba-2 RNAi-treated worms; time post wound [min:s]; scale bar 5 µm. (B) Quantification of EB1 comet number in either lateral epidermis or seam cells (n = 5) before wounding. (C) Quantification of EB1 recruitment at the wound site, at 10 min post-wounding (n = 10). Representative spinning disk images of Lifeact::mKate (D) kymograph analysis (E), intensity across the wound at 10 and 15 min (F) and quantification of the intensity of actin (G) and the area inside the actin ring (H) 15 min after wounding, in control and tubulin β encoding gene tbb-2 RNAi treated worms; ns p>0,05, *p<0.05, **p<0.01 and ****p<0.0001 non-parametric Mann-Whitney test.
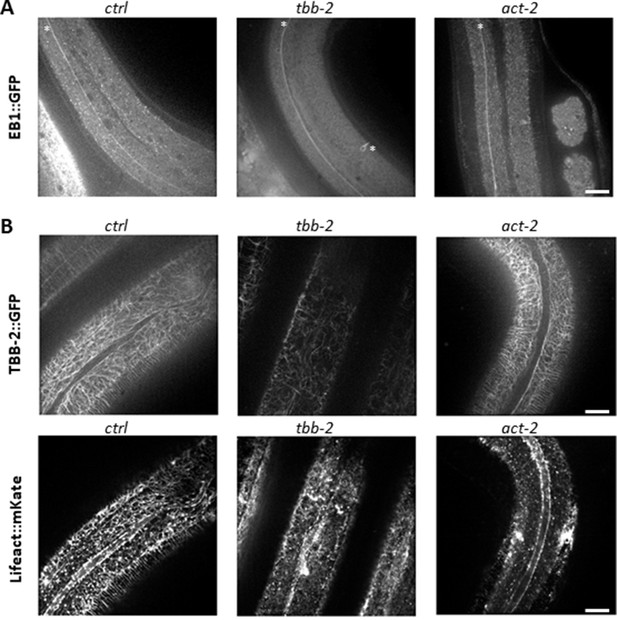
Tubulin isotypes required for non-centrosomal MT organisation in the lateral epidermis.
TBB-2 but not ACT-2 is required for the normal pattern of EB1 comets (A) and MTs (B). Representative spinning disk images of (A) EBP-2::GFP KI worms (white asterisks mark mechanosensory axons), or (B) TBB-2::GFP (top) and Lifeact::mKate (bottom) transgenes, after short temporal inactivation of control (sta-1; ctrl), tbb-2 and act-2 genes; scale bar 10 µm. bar 5 µm.
The MT + tip protein dynactin DNC-2 is recruited at the wound site.
Imaging DNC-2::GFP KI, Si col-62p::Lifeact::mKate2 strain; * indicates the wound site; [min:s]. Scale bar 5 µm.
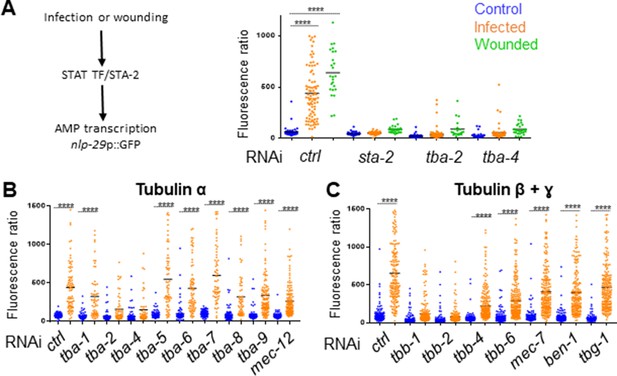
Specific tubulin isoforms are required for the activation of the immune response upon wounding and fungal infection.
Quantification of green fluorescence in a strain carrying the nlp-29p::GFP transcriptional reporter after RNAi against different tubulin α, β and γ genes in non-infected worms or after infection with D. coniospora or after wounding (blue, orange and green symbols, respectively). The ratio between GFP intensity and size (time of flight; TOF) is represented in arbitrary units. (A) Worms were fed on RNAi bacteria from the L4 stage and after 24 hr infected or wounded; sta-2(RNAi) is known to block the immune response (Dierking et al., 2011). (B, C) Worms sensitive to RNAi primarily in the adult epidermis were fed on RNAi clones from the L1 stage and infected with D. coniospora at the young adult stage. Mean are represented in black, numbers of worms in 5A: 47, 87, 25, 48, 46, 26, 66, 107, 21, 33, 52, 28; in 5B: 106, 82, 67, 59, 63, 57, 112, 51, 95, 65, 88, 76, 79, 65, 128, 58, 98, 121, 69, 138; in 5C: 228, 180, 199, 210, 201, 162, 145, 232, 220, 224, 147, 207, 196, 188, 133, 209. Only ****p<0.0001 is presented; ANOVA Bonferoni’s test. Graphs are representative of the results obtained from at least three independent replicates.
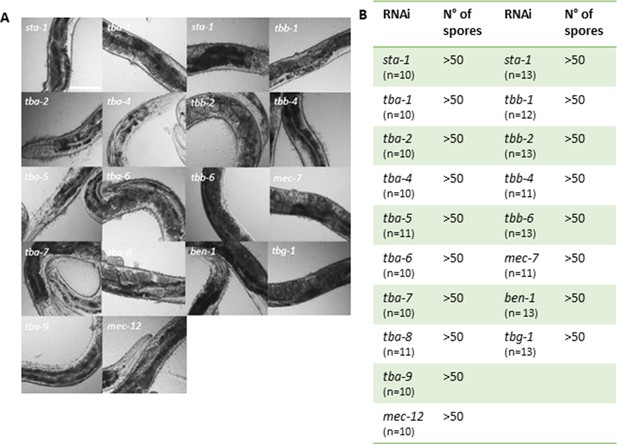
Worms are efficiently infected after knock-down of tubulin gene expression by RNAi.
(A) Representative white-field images of IG1327 worms after infection with D. coniospora for 18 hr. Before infection worms were fed with RNAi clones against different tubulin-α, β and γ isoforms. Each RNAi clone was tested four times. Scale bar 100 µm. (B) Table summarising the mean value of the number of spores adhering to the infected worms, after 18 h of D. coniospora infection. IG1327 rde-1(ne219) V; juIs346[col-19p::RDE-1, ttx-3p::GFP] III; frIs7[nlp-29p::GFP, col-12p::DsRed] IV.
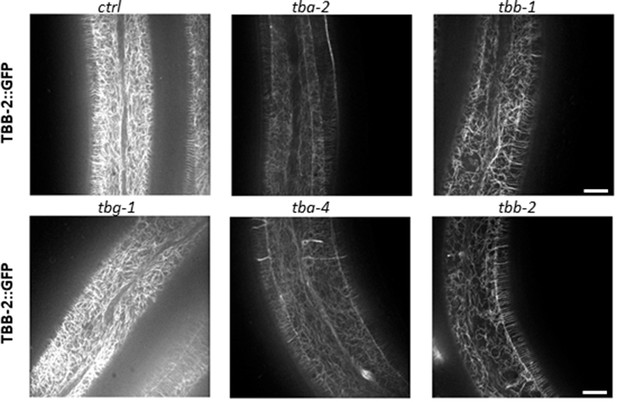
RNAi against tba-2, tba-4, tbb-1 and tbb-2, but not tbg-1 alters the pattern of MTs in the adult epidermis.
Representative spinning disk images of worms expressing TBB-2::GFP after short temporal inactivation of a ctrl (sta-1), tba-2, tba-4, tbb-1, tbb-2 and tbg-1 genes; scale bar 10 µm.
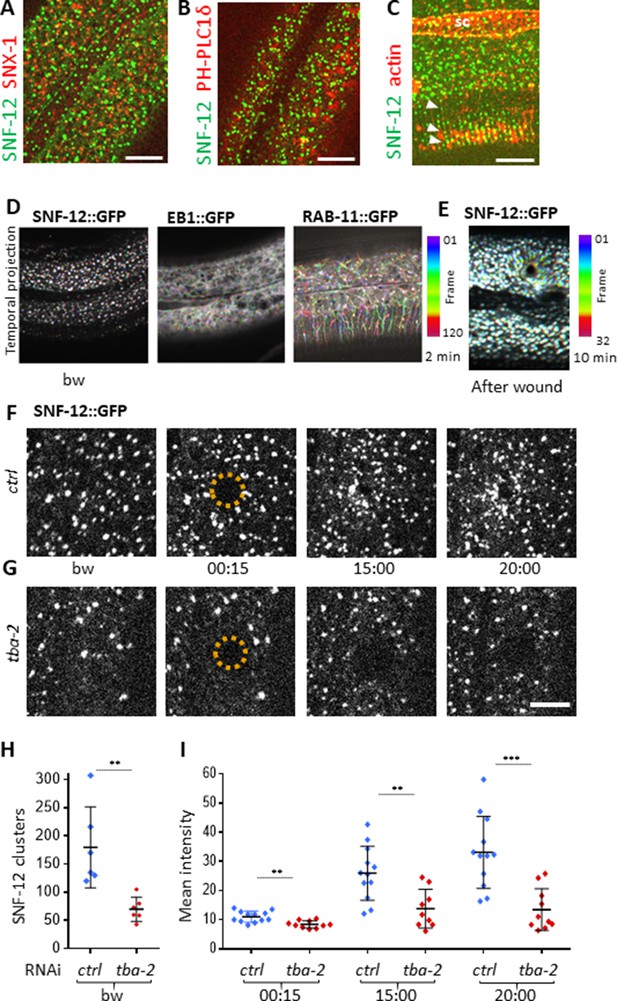
SNF-12 localizes to apical clusters that are recruited at the wound site in a MT-dependent way.
Representative spinning disk images of worms carrying col-12p::SNF-12::GFP as well as a red marker of early endosome (snx-1p::mRFP::SNX-1; A), membrane lipids (PIP2;ced-1p::mCherry::PH-PLC1δ; B) or actin (col-62p::Lifeact::mKate; C); scale bar 10 µm. (D) The different dynamics of SNF-12, EB1 and RAB-11 are represented with a temporal color-coded projection of 120 frames over 2 min (one fps) before wounding. (E) After wounding, SNF-12::GFP clusters move towards the wound as represented with a color coded temporal projection of 32 frames over 10 min before wounding and 40 after, 6.30 min after wounding. (F–G) The SNF-12 recruitment to the wound site seen in control animals (F) is abrogated upon tba-2 RNAi (G). The dashed circle is centred on the wound site; time post wound [min:s]; scale bar 5 µm. (H) Quantification of SNF-12 cluster number in the lateral epidermis (worm n = 6). (I) Quantification of SNF-12 recruitment at the wound site at different time points post wounding (wound n = 12 for control worms and 10 for tba-2 RNAi). **p<0.01 and ***p<0.001; non-parametric Mann-Whitney test.
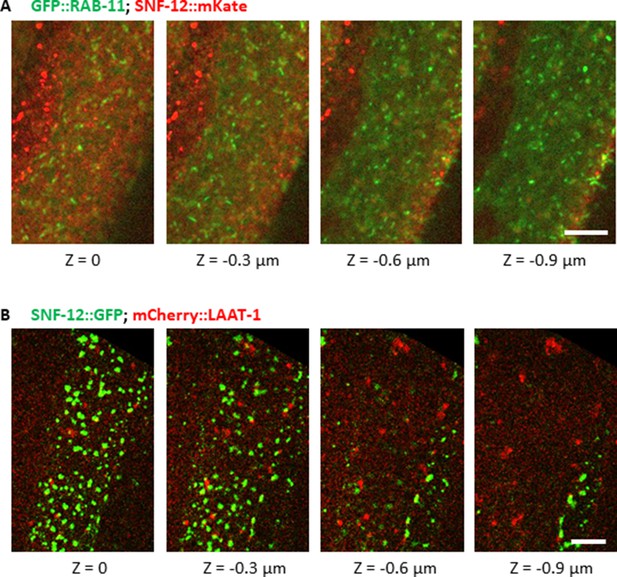
SNF-12 does not colocalise with RAB-11 or LAAT-1.
Colocalisation analyses to compare SNF-12 localization with known vesicular markers. Recycling endosomes (A) and lysosomes (B) are visualised as RAB-11 (A) and LAAT-1 (B) positive vesicles, respectively. SNF-12 did not colocalise with these markers. SNF-12 was in the same focal plane as the most apical RAB-11 recycling endosomes (A) and apical to LAAT-1-positive lysosomes (B). Z indicates the different focal planes. Representative images of dpy-7p::GFP::RAB-11; col-12p::SNF-12::mKate2 (A) and col-12p::SNF-12::GFP; ced-1p::LAAT-1::mCherry (B). Scale bar 5 µm.
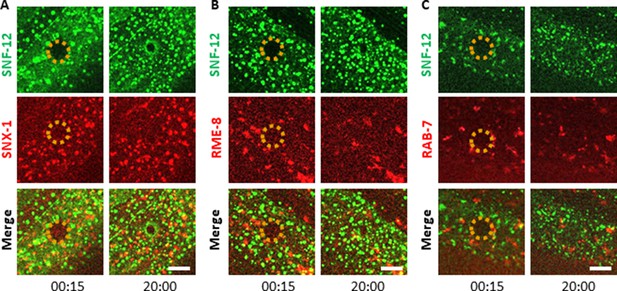
SNF-12 exhibits a specific dynamic behaviour upon wounding.
Unlike early endosomes (A-B) or late endosomes (C) visualized with SNX-1 (A), RME-8 (B) and RAB-7 (C) reporter proteins upon wounding, SNF-12 clusters are locally recruited to the wound site. Representative figures of col-12p::SNF-12::GFP; snx-1p::mRFP::SNX-1 (A), col-12p::SNF-12::GFP; RME-8::mRFP (B) and col-12p::SNF-12::GFP; ced-1p::mCherry::RAB-7 worms (C). The dashed circle is centred on the wound site; time post wound [min:s]; scale bar 5 µm.
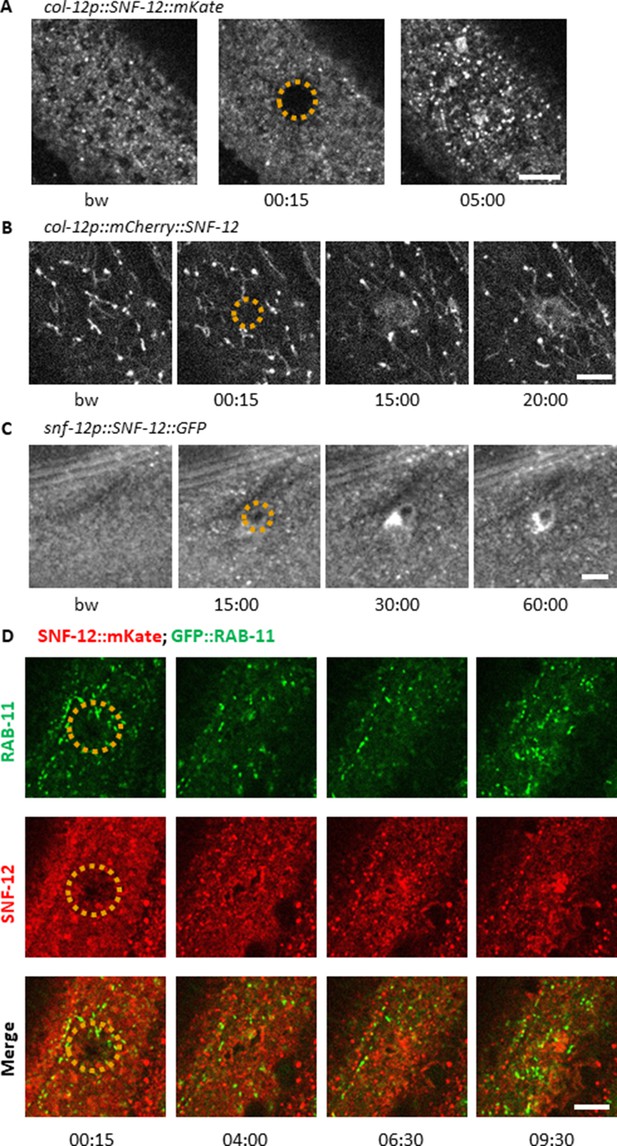
SNF-12 recruitment towards the wound site is observed using different chimeric reporter proteins.
Representative spinning disk images of worms carrying col-12p::SNF-12::mKate2 (IG1784; A), col‐12 p::mCherry::SNF‐12 (IG1270; B), snf-12p::SNF-12::GFP (IG1663; C) transgenes. In (B), the large aggregates and filaments that are observed in addition to the SNF-12-positive clusters are probably artefacts due to the known propensity of mCherry to aggregate. The dashed circle is centred on the wound site; time post wound [min:s]; scale bar 5 µm. (D) Recruitment of SNF-12 and RAB-11 at the wound results in clusters that do not greatly overlap.
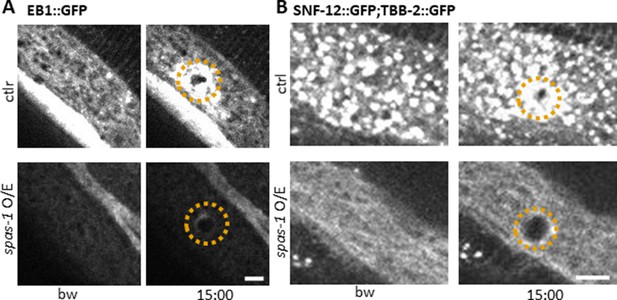
Spastin-1 overexpression drastically alters MT and SNF-12 dynamics before and after wounding.
Overexpression of the severing protein Spastin (SPAS-1) in the epidermis under the control of the dpy-7 promoter (spas-1 O/E) affects both EB1 and SNF-12 patterning and recruitment towards wounds. Representative images of worms carrying col-19p::EBP-2::GFP (A) and col-12p::SNF-12::GFP; col-19p::GFP::TBB-2 (B) without and with the dpy-7p::SPAS-1 construct (upper and lower panels, respectively) before and after wounding. The dashed circle is centred on the wound site; time post wound [min:sec]; scale bar, 5 µm. Note that the worms overexpressing Spastin are dumpy. In common with several dumpy mutants, they constitutively express the nlp-29p::GFP reporter (Dodd et al., 2018; Pujol et al., 2008b; Zugasti et al., 2014; Zugasti et al., 2016). Understanding the basis of this increased AMP gene expression will require further investigation.
SNF-12 dynamics in adult epidermis.
Imaging Is col-12p::SNF-12::GFP strain; [min:s].
SNF-12 vesicles are recruited at the wound site.
This movie follows SNF-12 recruitment from 6 min 54 s post wounding; it is a different wound from the one shown in Figure 6F. Imaging Is col-12p::SNF-12::GFP strain; * indicates the wound site; [min:s].
RAB-5 dynamics in adult epidermis.
Imaging juEx1919 dpy-7p::GFP::RAB-5 strain; [min:sec].
RAB-5 recruitment at the wound site.
Imaging juEx1919 dpy-7p::GFP::RAB-5 strain; * indicates the wound site; [min:sec].
RAB-11 dynamics in adult epidermis.
Imaging frSi13 dpy-7p::GFP::RAB-11 strain; [min:s].
RAB-11 recruitment at the wound site.
Imaging frSi13 dpy-7p::GFP::RAB-11 strain; * indicates the wound site; [min:s].
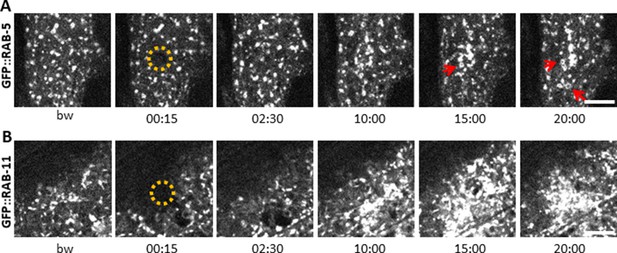
Early and recycling endosomes are recruited to the wound site.
Representative spinning disk images of worms carrying dpy-7p::GFP::RAB-5 (A) and dpy-7p::GFP::RAB-11 (B). A fraction of the early endosome marker RAB-5 localizes to donut-shaped structures (red arrows). Upon laser wounding these RAB-5-positive structures (A) as well as RAB-11-positive recycling endosomes (B) are recruited to the wound site within 10 min. The dashed circle is centred on the wound site; time post wound [min:s]; scale bar 5 µm.
Additional files
-
Source code 1
Orientation of fibers analysis.
- https://cdn.elifesciences.org/articles/45047/elife-45047-code1-v2.m
-
Supplementary file 1
MT-related genes identified in a genome-wide screen for regulators of AMP gene expression from Zugasti et al. (2016).
- https://cdn.elifesciences.org/articles/45047/elife-45047-supp1-v2.docx
-
Supplementary file 2
Key resources table.
- https://cdn.elifesciences.org/articles/45047/elife-45047-supp2-v2.pdf
-
Transparent reporting form
- https://cdn.elifesciences.org/articles/45047/elife-45047-transrepform-v2.pdf