Reducing respiratory syncytial virus (RSV) hospitalization in a lower-income country by vaccinating mothers-to-be and their households
Figures
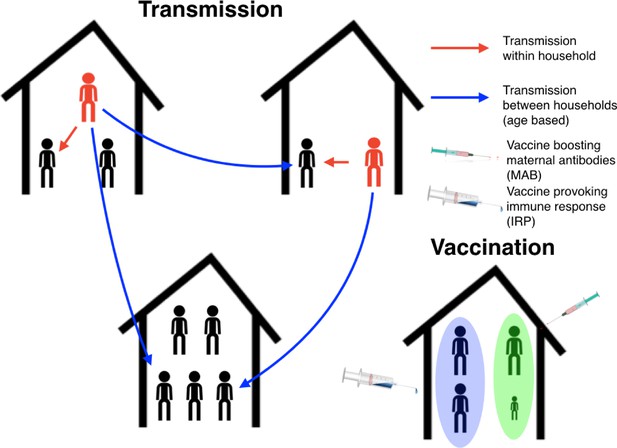
Schematic plot for the RSV transmission model and vaccination programme.
Infectious individuals (red character figures) transmit to other individuals inhabiting the same house, and to other individuals in other households based on the ages of the both the infector and infectee. Red and blue arrows represent possible realised infections over a short period of time. Bottom right household demonstrates the vaccination strategy; the mother has received a maternal antibody boosting (MAB) vaccine which increased transfer of protective antibodies to newborns (green background shading), meanwhile other household members have received an immune response provoking (IRP) vaccine (blue background shading).
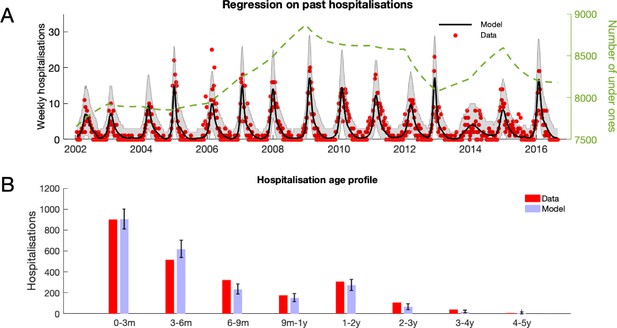
RSV hospitalisation at KCH: dynamics and age profile of hospitalised patients.
(A) Weekly RSV hospitalisations before implementation of vaccinations. Black curve gives mean prediction of RSV household transmission model after regression against weekly incidence data (red dots). Grey shaded area indicates the 99% prediction interval for the model. Also shown is the number of under ones in the population (dashed line). (B) Age profile of hospitalisations at KCH before vaccination. Error bars give 99% prediction intervals for model.
-
Figure 2—source data 1
Hospitalisation data, and model predictions, are given as MATLAB data files along with script for plotting figure.
- https://cdn.elifesciences.org/articles/47003/elife-47003-fig2-data1-v2.zip
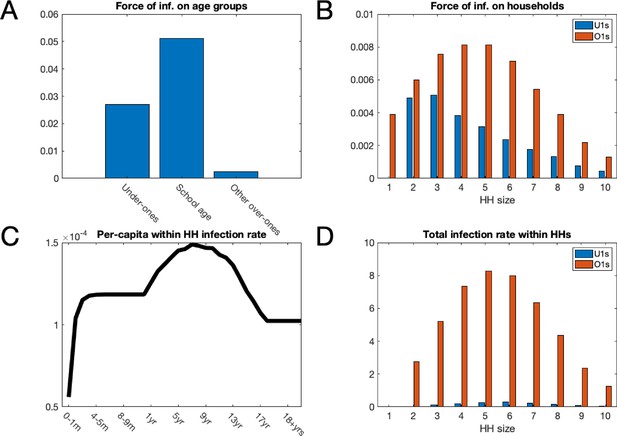
Mean force of infection (2002–2016) between households and mean infection rates within households.
(A) The mean force of infection (infectious contacts received per person per day) of RSV due to transmission from without the household on three age groups: under-ones, school age children and everyone else, including adults. (B) Mean force of infection due to transmission without the household on individuals inhabiting each household size. (C) The mean per-capita daily rate at which different age groups become infected with RSV from within their household. (D) The mean total daily rate of RSV infection within households of different sizes.
-
Figure 3—source data 1
The model predictions are given as MATLAB data files, along with the script for plotting figure.
- https://cdn.elifesciences.org/articles/47003/elife-47003-fig3-data1-v2.zip
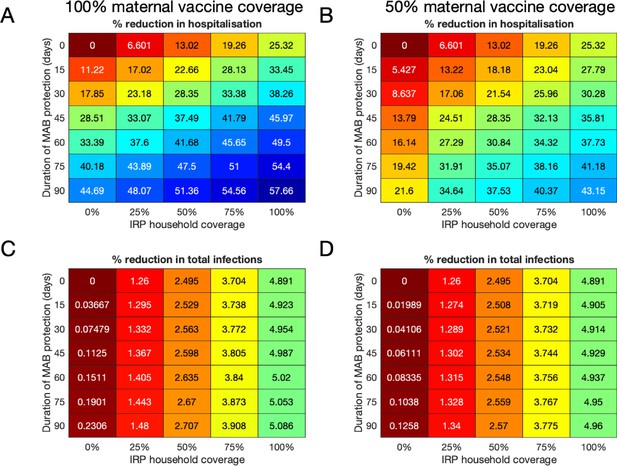
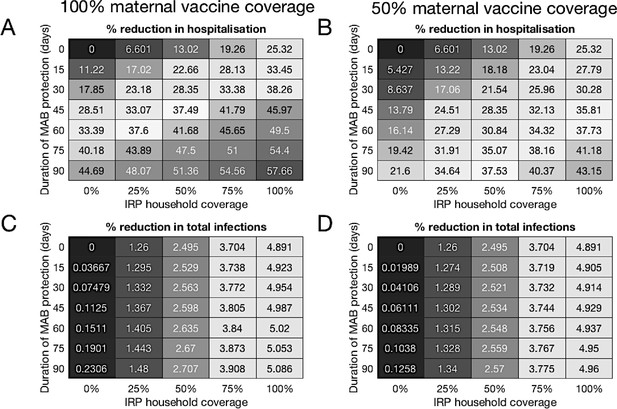
Median forecast effectiveness of RSV vaccination for different mixed strategies over a 10-year period for 100% maternal vaccine effective coverage (A and C) and 50% maternal vaccine effective coverage (B and D).
(A and B) Median percentage reduction in hospitalisations at KCH. (C and D) Percentage reduction in total RSV infections in the population.
-
Figure 4—source data 1
Reductions in hospitalisations and infections for each of the 500 forecasting simulations are given as MATLAB data files, along with script for plotting figure.
- https://cdn.elifesciences.org/articles/47003/elife-47003-fig4-data1-v2.zip
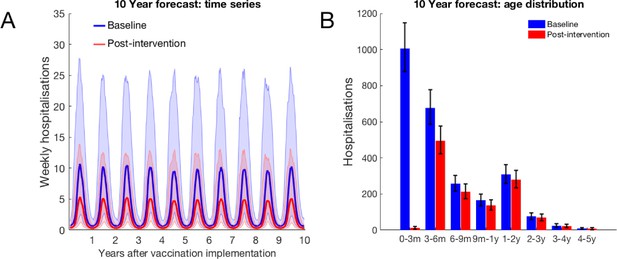
10-year forecast of RSV vaccination effectiveness for a mixed strategy of an MAB vaccine provided 75 days of additional RSV protection for newborns and a 75% IRP vaccine household coverage.
(A) Forecast weekly hospitalisations for a baseline of no vaccination (blue) and the mixed vaccination strategy (red). Shown are median forecast (curves) and 95% prediction intervals (background shading). (B) Forecast age distribution of total RSV hospitalisations at KCH. Median forecast (bars) and 95% prediction intervals (error bars).
-
Figure 5—source data 1
Hospitalisation predictions for each of 500 forecasting simulations is given as a MATLAB data file, along with a MATLAB function for combining the forecasting and Poisson hospitalisation rate uncertainties into a prediction interval and plotting script.
- https://cdn.elifesciences.org/articles/47003/elife-47003-fig5-data1-v2.zip
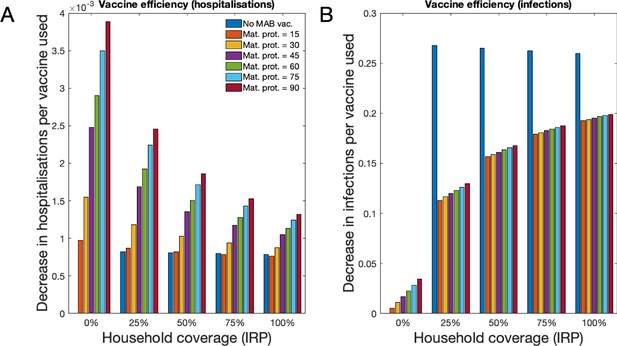
Forecast vaccination efficiency against hospitalisations and all infections, defined as number of cases averted per vaccine used (both MAB and IRP).
MAB vaccine coverage was 100% unless unavailable, however MAB protection duration varied (different coloured bars) and IRP household coverage was also varied. See Table 1 for a list of scenario. (A) Median avoided hospitalisations at KCH per vaccine over 500 simulations. (B) Median avoided RSV infections in population per vaccine over 500 simulations.
-
Figure 6—source data 1
A MATLAB script for converting 500 forecasting simulation outcomes into efficiency metrics, and plotting them.
- https://cdn.elifesciences.org/articles/47003/elife-47003-fig6-data1-v2.zip
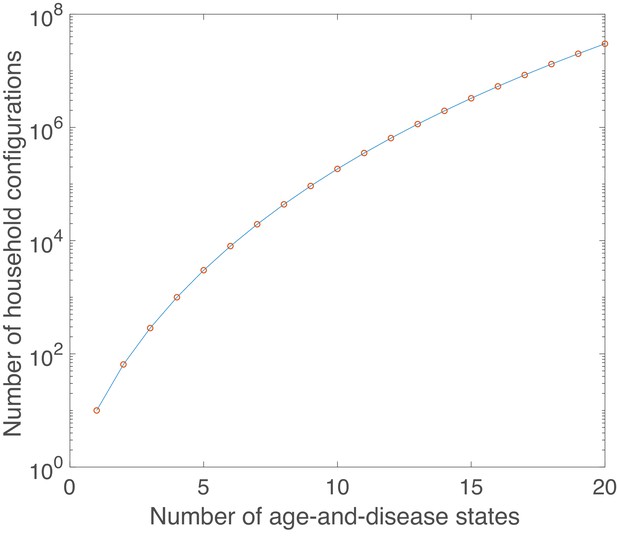
Growth in number of possible household configurations as complexity of the underlying age-and-disease state model grows.
Calculated for a maximum household size of 10.
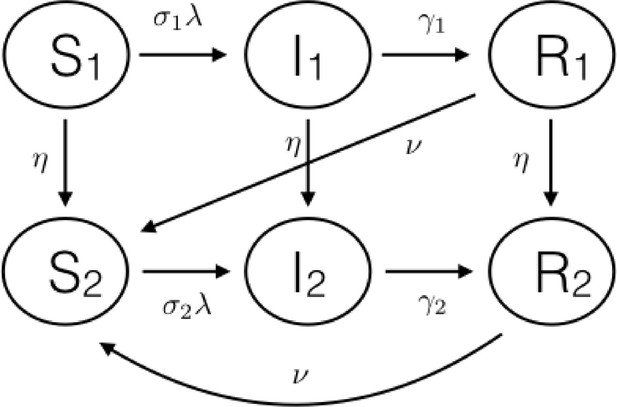
Schematic diagram of the basic age-and-disease state compartmental model for the individuals inside the households.
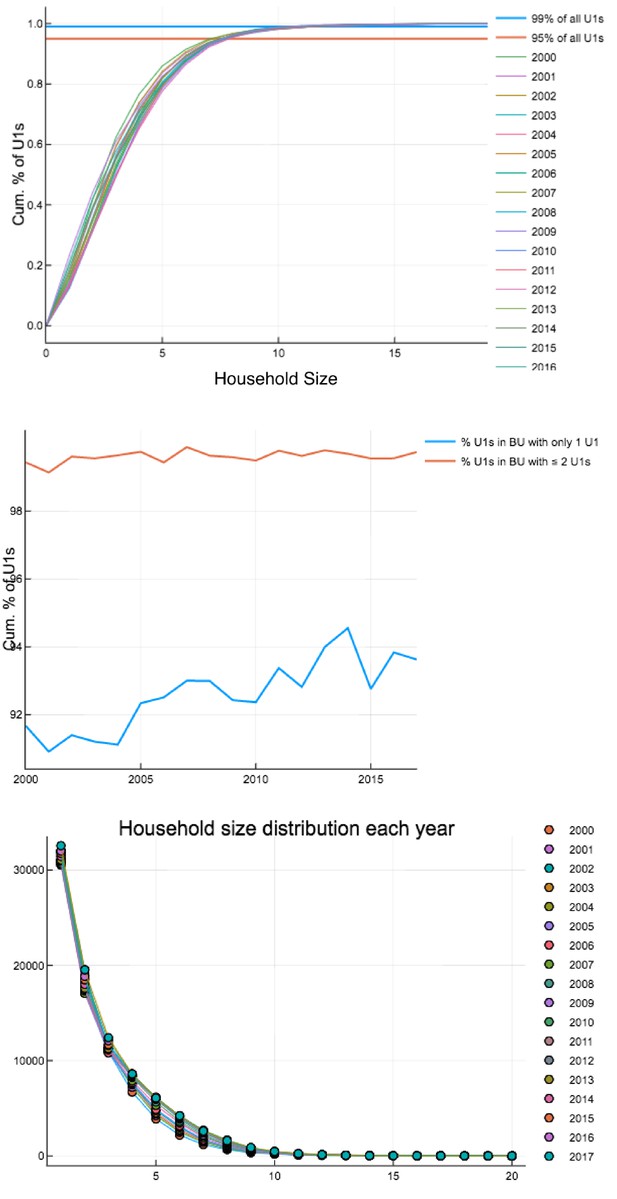
Household occupancy characteristics calculated on each 1 st Jan 2000–2017.
Top: Percentage of U1s in households of a certain size or smaller. Middle: Percentage of U1s in households with only one U1 and households with one or two U1s. Bottom: Household size distribution.
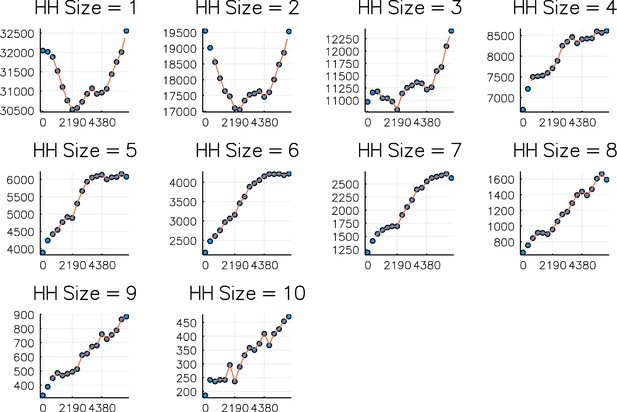
Comparison of numbers of households of sizes 1–10 on each 1 st Jan 2000–2017 (dots) against simulated values (curve).
Simulation is from Sept 2001 - Sept 2016. Horizontal axis is days since 1 st Jan 2000.
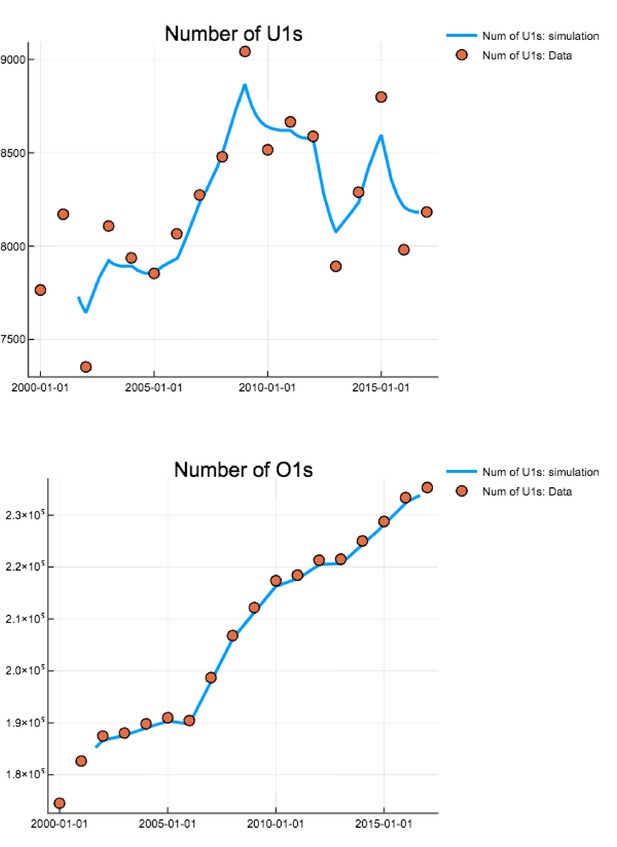
Comparison of total numbers of U1s and O1s on each 1 st Jan 2000–2017 (dots) against simulated values (curve).
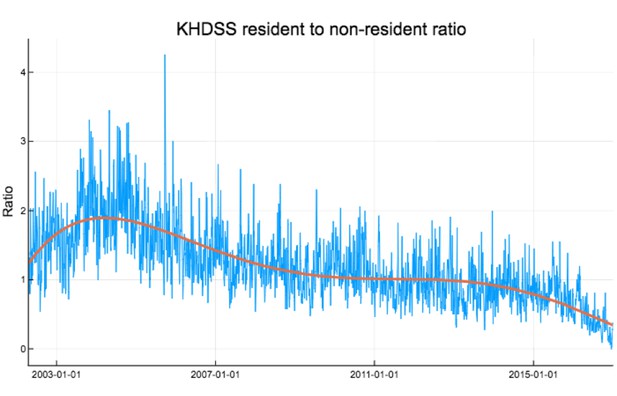
Ratio of KHDSS residents to non-residents weekly accessing KCH for confirmed RSV treatment.
Red curve is polynomial fit .
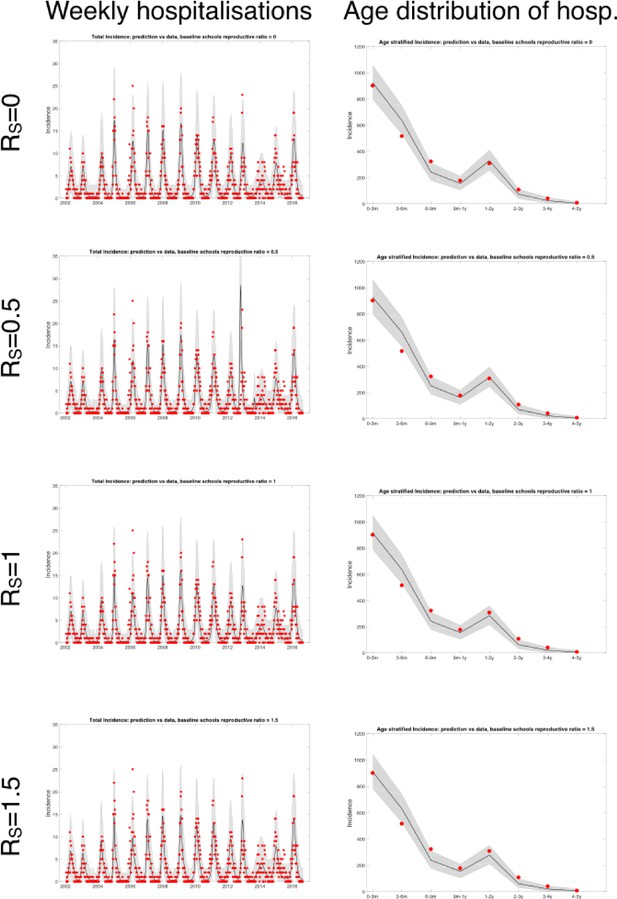
Plots of fitted weekly hospitalisations and the age distribution of hospitalisations for four scenarios (differing values of the schools based baseline ).
In each case, parameter inference was performed and the maximum likelihood estimators used.
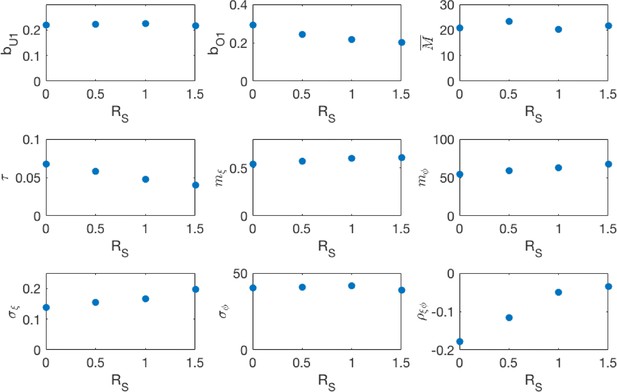
Maximum likelihood parameters for the different school transmission rate scenarios.
, are respectively the under-one and over-one mixing components of the community mixing rate matrix. is the rate at which a household member infectiously contacts each other household member. is the mean period of maternal protection after birth. is the mean vector of the random seasonality, and , and are respectively the standard deviations of the seasonal amplitude, seasonal phase and the correlation between the two, derived from the estimated covariance matrix .
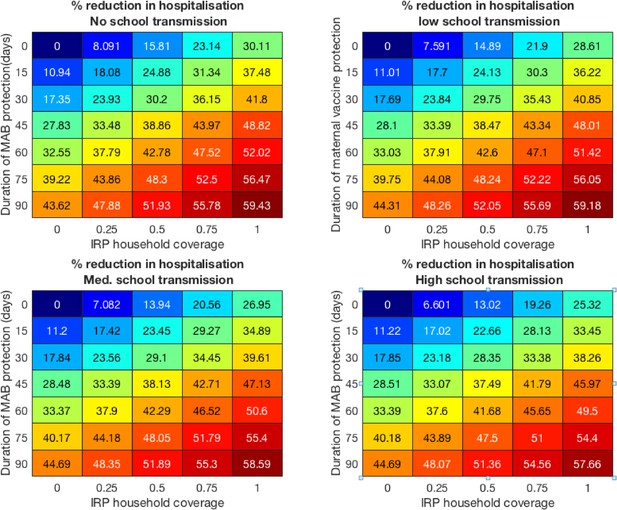
Vaccine effectiveness for the four school mixing scenarios at 100% MAB coverage.
Colorblind-friendly version of Figure 4 from main text.
Forecast effectiveness of RSV vaccination for different mixed strategies over a 10 year period for 100% maternal vaccine effective coverage (A and C) and 50% maternal vaccine effective coverage (B and D). (A and B) Percentage reduction in hospitalisations at KCH. (C and D) Percentage reduction in total RSV infections in the population.
Tables
Modelled vaccination scenarios.
Each combination of MAB vaccine effectiveness and coverage, with IRP vaccine coverage below was one scenario. The baseline scenario being no effective MAB vaccine and 0% coverage of IRP vaccine.
| Description | Range |
|---|---|
| Additional period of protection from RSV at birth due to maternal antibody boosting (MAB) vaccine (). | 0 (no vaccine), 15, 30, 45, 60, 75, 90 days |
| Coverage of mothers with MAB vaccine | 50%, 100% |
| Coverage of households with newborns with immune response provoking (IRP) vaccination () | 0%, 25%, 50%, 75%, 100% |
Parameters from literature and chosen for model.
| Parameter | Description | Value | Data source |
|---|---|---|---|
| Susceptibility (O1s) | 0.75 | Henderson et al., 1979 | |
| relative infectiousness (O1s) | 0.5 | Kinyanjui et al., 2015 | |
| Rate of waning of immunity | two per year | Agoti et al., 2012 | |
| Rate of recovery for under-ones | 1/9 per day | Hall et al., 1976 | |
| Rate of recovery for over-ones | 1/4 per day | Hall et al., 1976 | |
| Community transmission rate at schools | 0,1/3,2/3,1 per day | range | |
| Ageing rate for U1s | one per year | model choice | |
| Base external infection rate (whole population) | 10 per day | model choice |
Inferred parameters.
| Parameter | Description | Value |
|---|---|---|
| Community transmission rate for U1s | 0.22 [0.18,0.27] per day | |
| Community transmission rate for O1s | 0.20 [0.18,0.21] per day | |
| Transmission rate to each other member of household | 0.040 [0.032, 0.048] per day | |
| Mean duration of maternal protection at birth | 21.6 [17.2, 26.1] days | |
| Mean amplitude of log-seasonality | 0.61 [0.51, 0.72] | |
| Mean timing of log-seasonality peak (phase) | 67.7 [40.2, 77.7] days | |
| Std. amplitude of log-seasonality | 0.20 [0.098,0.31] | |
| Std. timing of log-seasonality peak (phase) | 38.7 [30.0, 48.5] days | |
| Corr. coefficient between log-seasonal amplitude and phase | −0.035 [-0.12, 0.072] |
Parameters estimated from KHDSS data.
| Parameter | Description | Value | Data source |
|---|---|---|---|
| μ(n, t) | Birth/turnover rate for households of size n on day t | Varies, see above | KHDSS |
| r(n, t) | Rate of change of numbers of households of size n on day t | Varies, see above | KHDSS |
| PH→A,t | Conditional age distribution given household config. on day t | Varies, see above | KHDSS |
| PA→H,t | Conditional household config. distribution given age category on day t | Varies, see above | KHDSS |
Age-dependent hospitalisation probabilities per infection derived from Kinyanjui et al., 2015.
| Age category | Probability of hospitalisation per infection |
|---|---|
| 0-1 month | 0.10 |
| 1-2 month | 0.10 |
| 2-3 month | 0.063 |
| 3-4 month | 0.059 |
| 4-5 month | 0.054 |
| 5-6 month | 0.025 |
| 6-7 month | 0.019 |
| 7-8 month | 0.022 |
| 8-9 month | 0.012 |
| 9-10 month | 0.016 |
| 10-11 month | 0.013 |
| 11-12 month | 5.1x10−3 |
| 1-2 years old | 2.6x10−3 |
| 2-3 years old | 7.5x10−4 |
| 3-4 years old | 2.2x10−4 |
| 4-5 years old | 3.8x10−5 |
Model parameters inferred from hospitalisation data.
| bU1 | Community transmission rate for U1s | 0.22 [0.18,0.27] per day |
|---|---|---|
| bO1 | Community transmission rate for O1s | 0.20 [0.18,0.21] per day |
| τ | Transmission rate to each other member of household | 0.040 [0.032, 0.048] per day |
| M | Mean duration of maternal protec- tion at birth | 21.6 [17.2, 26.1] days |
| mξ | Mean amplitude of log-seasonality | 0.61 [0.51, 0.72] |
| mφ | Mean timing of log-seasonality peak (phase) | 67.7 [40.2, 77.7] days |
| σξ | Std. amplitude of log-seasonality | 0.20 [0.098,0.31] |
| σφ | Std. timing of log-seasonality peak (phase) | 38.7 [30.0, 48.5] days |
| ρξφ | Corr. coefficient between log- seasonal amplitude and phase | -0.035 [-0.12, 0.072] |