TRF1 averts chromatin remodelling, recombination and replication dependent-break induced replication at mouse telomeres
Figures
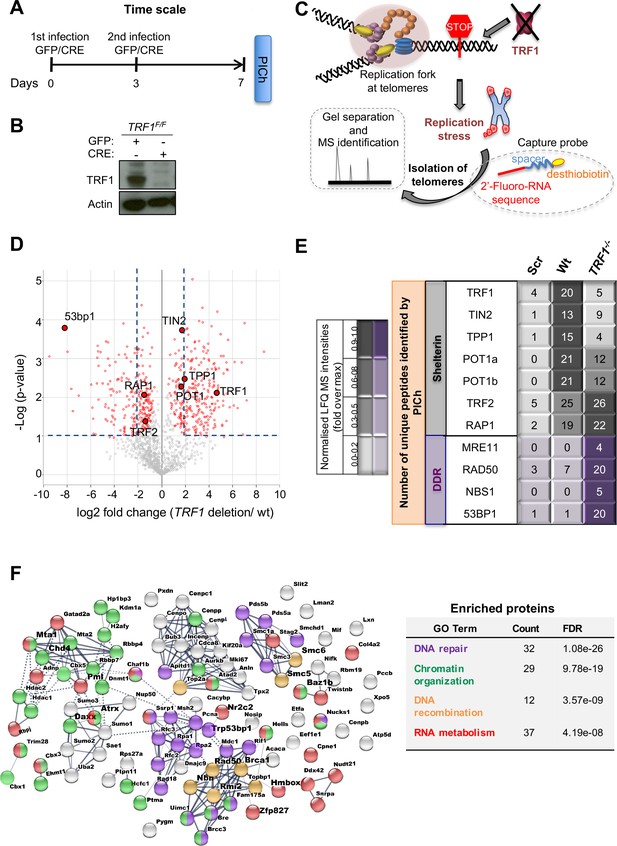
Proteomics of isolated chromatin segments (PICh) of TRF1 depleted mouse telomeres.
(A) Overview of experimental timeline aimed at performing PICh experiment after induction of TRF1 deletion. TRF1F/F MEFs were infected twice (day 0 and 3) with adenovirus containing either GFP-control or CRE and collected at day 7 for PICH experiments. (B) Western blot showing deletion of TRF1 in MEFs after infections with CRE Adenovirus, at day 7 as in A. (C) Schematic representation of the PICh analysis performed to detect chromatin changes occurring at telomeres upon TRF1 deletion. (D) Volcano Plot based on LFQ intensities of proteins. Cut off for differential expression were set to log2 fold change (TRF1deletion/wt)> |2| and -Log (p-value) > 1. (E) Table listing shelterin components and some of the DNA damage response (DDR) factors identified. The corresponding number of unique peptide isolated is indicated for each factor of interest. Relative LFQ intensity abundance profiles were visualised in the form of a heat-map, by scaling each protein intensity to the maximum intensity across conditions. Light to darker colours indicate increasing relative protein abundance. (F) Connectivity map for proteins recruited at telomeres upon TRF1 deletion using string-db.org software. Solid lines, represents strong direct interactions, while dashed lines represent no evidence for direct interaction. In violet, DNA damage and repair proteins; in orange, factors belonging to DNA repair specifically involved in DNA recombination process; while in green and red, important factors for chromosome maintenance and factors involved in RNA metabolism, respectively. Source data are provided as a Source Data File.
-
Figure 1—source data 1
TRF1F/F MEFs western blot.
Related to Figure 1B.
- https://cdn.elifesciences.org/articles/49817/elife-49817-fig1-data1-v4.xlsx
-
Figure 1—source data 2
PICh data (TRF1F/F telo vs TRF1-/- telo).
Related to Figure 1D.
- https://cdn.elifesciences.org/articles/49817/elife-49817-fig1-data2-v4.xlsx
-
Figure 1—source data 3
PICh data.
Related to Figure 1F.
- https://cdn.elifesciences.org/articles/49817/elife-49817-fig1-data3-v4.xlsx
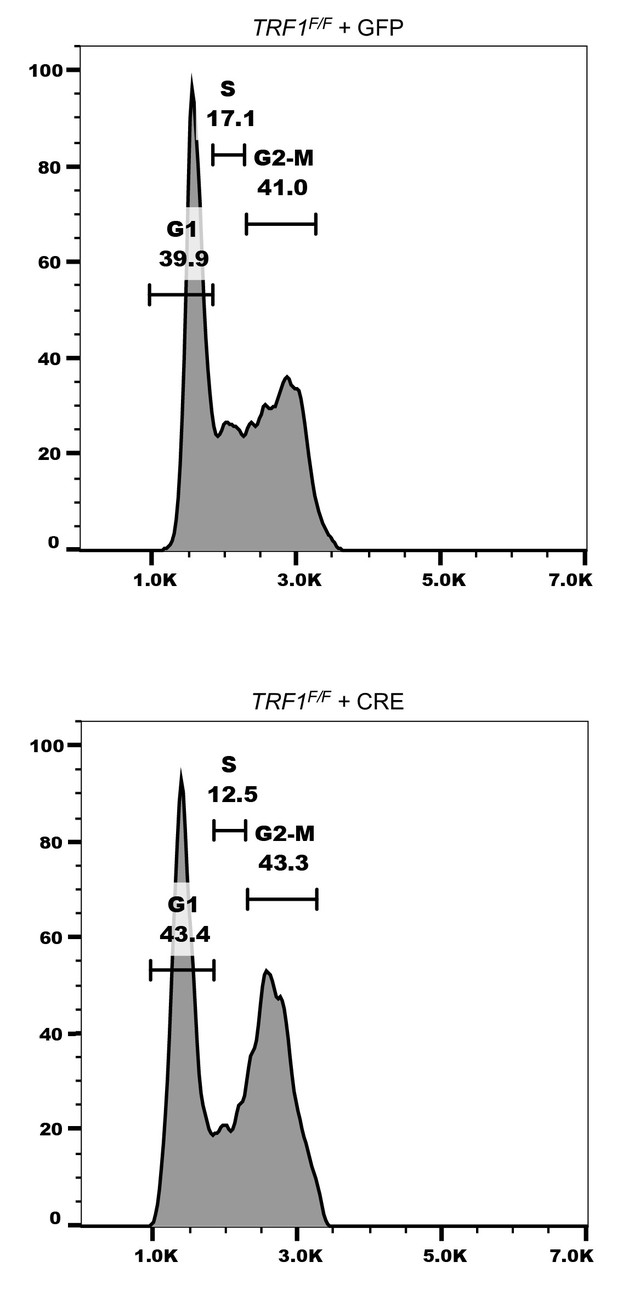
TRF1 deficient MEFs show no drastic changes in their cell cycle.
The cell cycle status of TRF1F/F MEFs infected with GFP or CRE adenovirus was examined via flow cytometry. DNA was stained with propidium iodide and cells were incubated with EdU to mark newly synthesized DNA. Gates delineate the different stages of the cell cycle. The present graph displays data from 10,000 cells of 2 independent biological replicates.
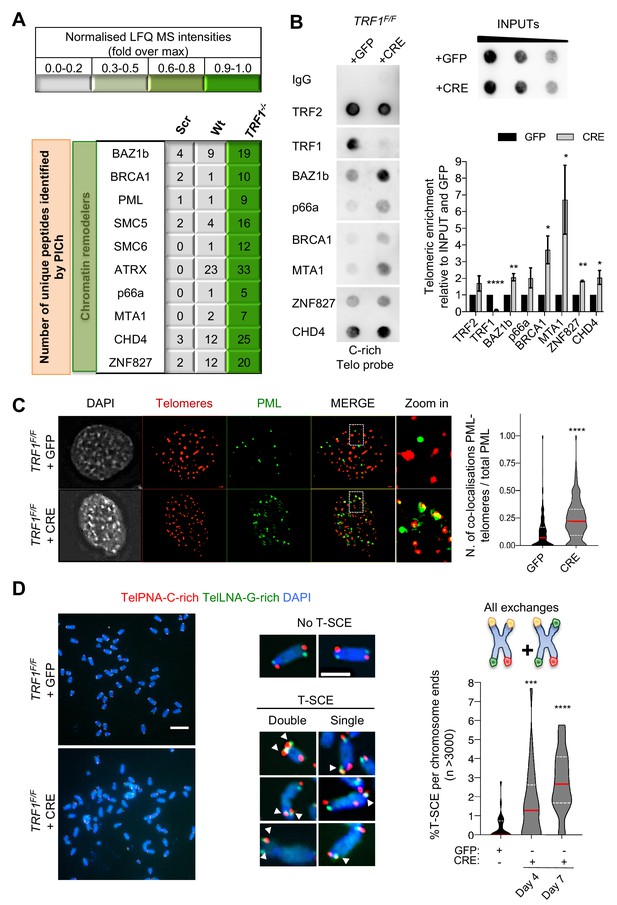
Recombination factors are recruited at TRF1 depleted telomeres.
(A) Table listing chromatin remodellers identified. The corresponding number of unique peptide isolated is indicated for each factor of interest. Same as in Figure 1, light to darker colours indicate increasing relative protein abundance. (B) Validation of chromatin remodeller factors by ChIP-dot blot analysis in wt (+GFP) and TRF1-/- (+CRE) conditions using ChIP grade antibodies against chosen factors after chromatin preparation from MEFs. The blot was revealed with a DIG-Tel-C-rich probe. ChIP signals were normalised to DNA input and GFP control. Data are represented as telomeric enrichment of proteins relative to GFP ± SEM of at least three independent biological replicates. P values, two-tailed student t-test (*, p<0.05; **, p<0.01; ****, p<0.0001). (C) Representative image of Immunofluorescence showing co-localisation of Telomeres (red) with PML (green) in MEFs nuclei (DAPI) treated with GFP and CRE. Data from two independent biological replicates are represented as number of Telomeres-PML co-localising foci divided by the total number of PML present per nucleus (n = 300 nuclei) with a violin plot, where the median is underlined in red and quartiles in white. P values, two-tailed student t-test (****, p<0.0001). Source data are provided as a Source Data File. (D) Representative images of the chromosome-oriented CO-FISH assay with denaturation, used to score for telomeric T-SCEs in TRF1F/F MEFs infected with GFP or CRE. Telomeres are labelled with TelPNA-C-rich-Cy3 (red) and TelLNA-G-rich-FAM (green), while chromosomes are counterstained with DAPI (blue). Scale bar, 10 µm. Enlarged intersections show the difference between a chromosome with No T-SCE (top) and a chromosome with T-SCE (bottom). T-SCE images show double T-SCEs (left) and single chromatid events (right). Scale bar, 2 µm. For quantification, T-SCE was considered positive when involved in a reciprocal exchange of telomere signal with its sister chromatid (both telomeres yellow) and for asymmetrical exchanges at single chromatid (one telomere yellow). Data are indicated as % of T-SCE per sister telomere (n = > 3000 chromsome ends) and are represented with a violin plot, where the median is underlined in red and quartiles in white. P value, two-tailed student t-test (****, p<0.0001).
-
Figure 2—source data 1
Control specificity of proteomic binding (TRF1-/- telo vs TRF1-/- scbl).
Related to Figure 2A.
- https://cdn.elifesciences.org/articles/49817/elife-49817-fig2-data1-v4.xlsx
-
Figure 2—source data 2
ChIP quantification in TRF1F/F MEFs.
Related to Figure 2B.
- https://cdn.elifesciences.org/articles/49817/elife-49817-fig2-data2-v4.xlsx
-
Figure 2—source data 3
Quantification of APBs in TRF1F/F MEFs.
Related to Figure 2C.
- https://cdn.elifesciences.org/articles/49817/elife-49817-fig2-data3-v4.xlsx
-
Figure 2—source data 4
T-SCEs quantification in TRF1F/F MEFs.
Related to Figure 2D.
- https://cdn.elifesciences.org/articles/49817/elife-49817-fig2-data4-v4.xlsx
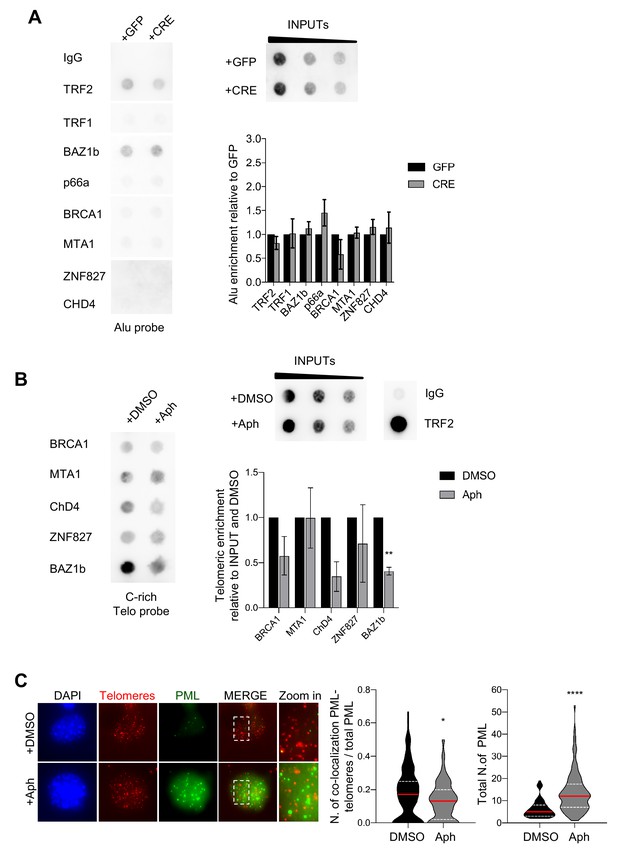
Validation of telomeric ChIP with ALU probe and effect of APH on telomeric chromatin and APBs.
(A) Control for Figure 2B, dot-blot for validation of chromatin remodellers factor specifically recruited at TRF1 depleted telomeres. The blot was revealed with a DIG-Alu probe (left panel). ChIP signals were normalised to DNA input (top, right panel) and GFP control and data are represented as relative Alu enrichment ± SEM of 3 independent biological replicates. P values, two-tailed student t-test. Source data are provided as a Source Data File. (B) Detection of chromatin remodeler factors by ChIP-dot blot analysis in wt (+DMSO) and Aphidicolin treated cells (+Aph) conditions using ChIP grade antibodies in MEFs chromatin (left panel). The blot was revealed with a DIG-Tel-C-rich probe. ChIP signals were normalised to DNA input (top middle panel) and GFP control. IgG and TRF2 antibodies were used respectively as negative and positive control for the ChIP experiment (top right panel). Data are represented as telomeric enrichment of proteins relative to DMSO ± SEM of 2 independent biological replicates. P values, two-tailed student t-test (**, p<0.01). (C) Representative image of Immunofluorescence showing co-localisation of Telomeres (red) with PML (green) in MEFs nuclei (DAPI) treated with DMSO or Aphidicolin. Data are represented as number of Telomeres-PML co-localising foci divided by the total number of PML present per nucleus (n > 100 nuclei) and three independent biological replicates are shown in a violin plot, where the median is underlined in red and quartiles in white (left graph). Number of total PML foci per nucleus from three independent biological replicates are shown in a violin plot, where the median is underlined in red and quartiles in white (right graph). P values, two-tailed student t-test (*, p<0.05; ****, p<0.0001). Source data are provided as a Source Data File.
-
Figure 2—figure supplement 1—source data 1
Alu probe control for ChIP quantification in TRF1F/F MEFs.
Related to Figure 2—figure supplement 1A.
- https://cdn.elifesciences.org/articles/49817/elife-49817-fig2-figsupp1-data1-v4.xlsx
-
Figure 2—figure supplement 1—source data 2
ChIP quantification in MEFs treated with APH.
Related to Figure 2—figure supplement 1B.
- https://cdn.elifesciences.org/articles/49817/elife-49817-fig2-figsupp1-data2-v4.xlsx
-
Figure 2—figure supplement 1—source data 3
Quantification of APBs in MEFs treated with APH.
Related to Figure 2—figure supplement 1C.
- https://cdn.elifesciences.org/articles/49817/elife-49817-fig2-figsupp1-data3-v4.xlsx
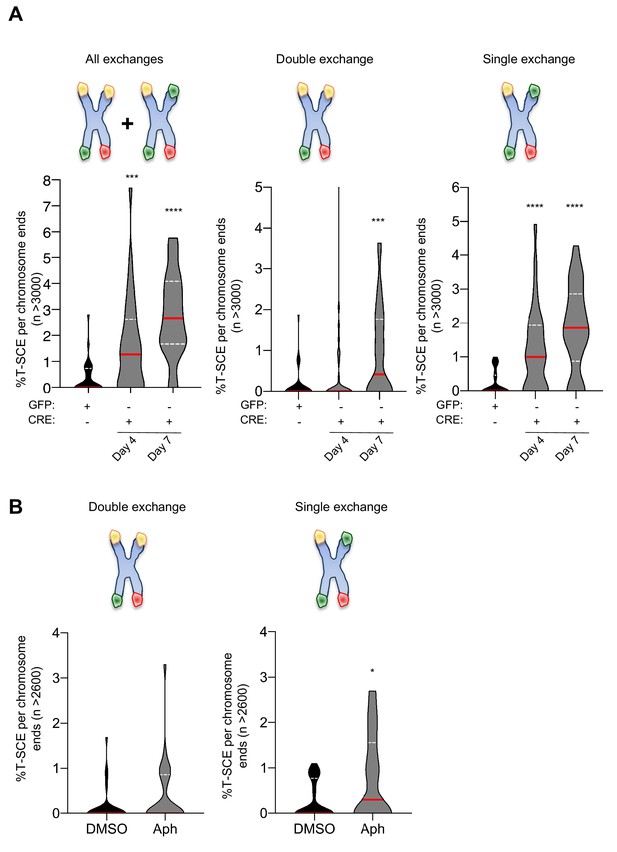
TRF1 loss and APH replication stress induce different types of telomeric recombination.
(A) Time course quantification of the different classes of T-SCEs using denaturing CO-FISH. TRF1F/F MEFs were collected 4 days and 7 days post-infection with CRE-adenovirus or GFP- (control). The different types of exchanges were classified into three different categories: all exchanges (single + double); double exchanges (reciprocal, both chromatids); single exchanges (asymmetrical, single chromatid). Violin plots are representing as % of T-SCE per chromosome ends (n = at least 3000 events were scored), where the median is underlined in red and quartiles in white. P value, two-tailed student t-test (****, p<0.0001; ***, p<0.001; n.s. = non significant). (B) Quantification of telomeric T-SCEs in wt MEFs treated with DMSO or Aphidicolin. Telomeric exchanges are classified as double exchanges (reciprocal, both chromatids-left graph) and single exchanges (asymmetrical, single chromatid-right graph). Violin plots represent % of T-SCE per chromosome ends (n = at least 1500 events were scored), where the median is underlined in red and quartiles in white. P value, two-tailed student t-test (*, p<0.05).
-
Figure 2—figure supplement 2—source data 1
T-SCEs quantification in TRF1F/F MEFs.
Related to Figure 2—figure supplement 2A.
- https://cdn.elifesciences.org/articles/49817/elife-49817-fig2-figsupp2-data1-v4.xlsx
-
Figure 2—figure supplement 2—source data 2
T-SCEs quantification of MEFs treated with APH.
Related to Figure 2—figure supplement 2B.
- https://cdn.elifesciences.org/articles/49817/elife-49817-fig2-figsupp2-data2-v4.xlsx
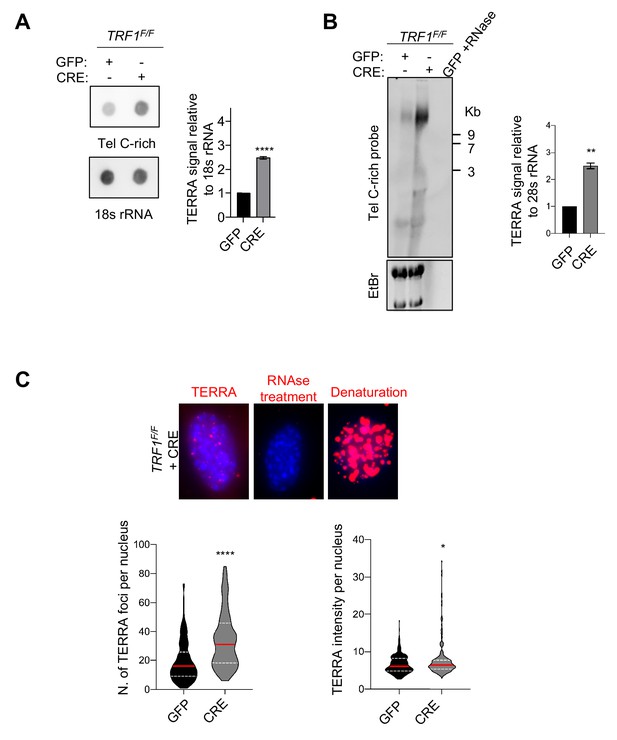
TRF1 depletion causes TERRAs upregulation.
(A) RNA dot blot analysis in wt and TRF1 deleted MEFs. The blot was revealed with a DIG-Tel-C-rich probe or 18 s rRNA as a control. TERRA signals were normalised to 18 s rRNA and GFP control ± SEM of at least three independent biological replicates. P values, two-tailed student t-test (****, p<0.0001). (B) TERRA detection by Northern blotting upon TRF1 deletion. The blot was revealed with a DIG-Tel-C-rich probe (upper part). Ethidium bromide (EtBr) staining (bottom) of rRNAs was used as loading control. TERRA signals were normalized to 28 s rRNA signal from EtBr staining ± SEM of 2 independent biological replicates. P values, two-tailed student t-test (**, p<0.01). (C) Representative images of TERRA-FISH experiment (top panel) showing the difference between cells stained with TERRA (red), negative control with RNAse A treatment and positive control after denaturation. TERRA-FISH quantification (bottom panel) in wt (+GFP) and TRF1-/- (+CRE) conditions. Violin plots are representing the number of TERRA foci (left) and TERRA intensity (right) (n = 250) per nucleus, where the median is underlined in red and quartiles in white, two-tailed student t-test (****, p<0.0001); Mann-Whitney test used for TERRA intensity quantification (*, p<0.05).
-
Figure 3—source data 1
Quantification of Telomeric RNA molecules by dot-blot in TRF1F/F MEFs.
Related to Figure 3A.
- https://cdn.elifesciences.org/articles/49817/elife-49817-fig3-data1-v4.xlsx
-
Figure 3—source data 2
Quantification of TERRAs by Northern.
Related to Figure 3B.
- https://cdn.elifesciences.org/articles/49817/elife-49817-fig3-data2-v4.xlsx
-
Figure 3—source data 3
Quantification of number of TERRA foci.
Related to Figure 3C.
- https://cdn.elifesciences.org/articles/49817/elife-49817-fig3-data3-v4.xlsx
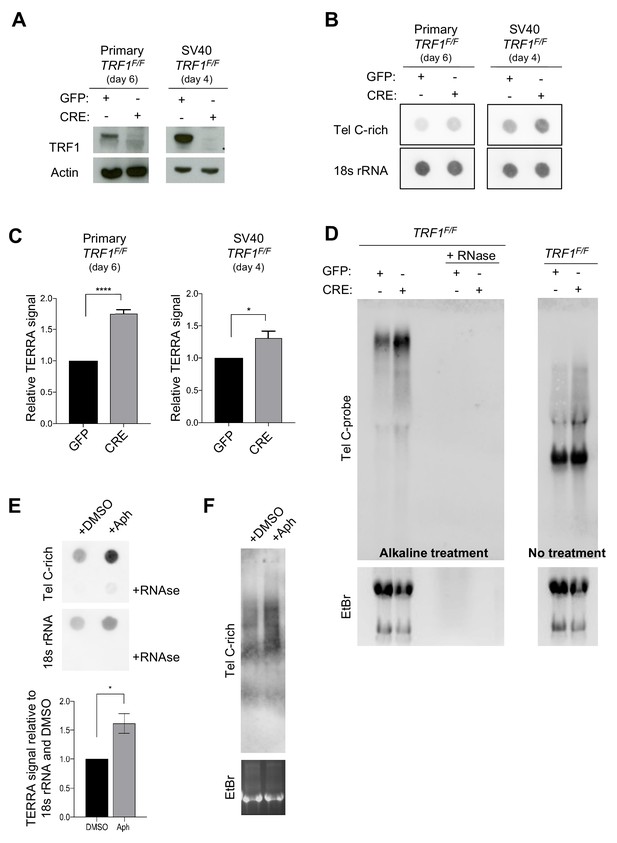
TRF1 deletion and APH replication stress cause increased TERRA levels in immortalised MEFs (day4) and also in primary MEFs (day 6).
(A) Western blotting showing protein expression in wt and TRF1 deficient primary MEFs (P4) 6 days post-infection with GFP- or CRE-Adenovirus (left panel) and in SV40-immortalised MEFs, 4 days post-infection (right panel). (B) RNA dot-blot analysis upon TRF1 deletion showing increased TERRA signals in CRE-infected conditions compared to control GFP-. The blot was revealed with a DIG-Tel-C-rich probe or 18 s rRNA as a control. (C) Quantification of B. Data are shown as TERRA signal relative to GFP condition ± SEM of 4 independent biological replicates. P values, two-tailed student t-test (*, p<0.05; ****, p<0.0001) (D) TERRA detection by Northern blotting upon TRF1 deletion showing increased High Molecular Weights (HMW) RNA molecules upon alkaline treatment (left blot). In native conditions, HMW-TERRAs are not detected and no significative difference is observed for low molecular weight species (right blot). The blots were revealed with a DIG-Tel-C-rich probe (upper part). Ethidium bromide (EtBr) staining (bottom) of rRNAs was used as loading control. Source data are provided as a Source Data File. (E) RNA dot-blot analysis in wt MEFs cells upon Aphidicolin treatment showing increased TERRA signals compared to control DMSO. The blot was revealed with a DIG-Tel-C-rich probe or 18 s rRNA as a control (top panel). Quantification of Data are shown as TERRA signal relative to DMSO condition ± SEM of 4 independent biological replicates (bottom panel). P values, two-tailed student t-test (*, p<0.05). (F) TERRA detection by Northern blotting upon Aphidicolin treatment showing increased High Molecular Weights (HMW) RNA molecules upon alkaline treatment. The blot was revealed with a DIG-Tel-C-rich probe (upper part). Ethidium bromide (EtBr) staining (bottom) of rRNAs was used as loading control. Source data are provided as a Source Data File.
-
Figure 3—figure supplement 1—source data 1
WB of TRF1F/F primary MEFs and immortalized after 4 days of CRE.
Related to Figure 3—figure supplement 1A.
- https://cdn.elifesciences.org/articles/49817/elife-49817-fig3-figsupp1-data1-v4.xlsx
-
Figure 3—figure supplement 1—source data 2
Telomeric RNA molecules by dot-blot in primary TRF1F/F MEFs and immortalized after 4 days of CRE.
Related to Figure 3—figure supplement 1B.
- https://cdn.elifesciences.org/articles/49817/elife-49817-fig3-figsupp1-data2-v4.xlsx
-
Figure 3—figure supplement 1—source data 3
Quantification of Telomeric RNA molecules by dot-blot in primary TRF1F/F MEFs and immortalized after 4 days of CRE.
Related to Figure 3—figure supplement 1C.
- https://cdn.elifesciences.org/articles/49817/elife-49817-fig3-figsupp1-data3-v4.xlsx
-
Figure 3—figure supplement 1—source data 4
TERRAs by Northern in TRF1F/F MEFs.
Related to Figure 3—figure supplement 1D.
- https://cdn.elifesciences.org/articles/49817/elife-49817-fig3-figsupp1-data4-v4.xlsx
-
Figure 3—figure supplement 1—source data 5
Quantification of Telomeric RNA molecules by dot-blot in wt MEFs treated with APH.
Related to Figure 3—figure supplement 1E.
- https://cdn.elifesciences.org/articles/49817/elife-49817-fig3-figsupp1-data5-v4.xlsx
-
Figure 3—figure supplement 1—source data 6
TERRAs by Northern in wt MEFs treated with APH.
Related to Figure 3—figure supplement 1F.
- https://cdn.elifesciences.org/articles/49817/elife-49817-fig3-figsupp1-data6-v4.xlsx
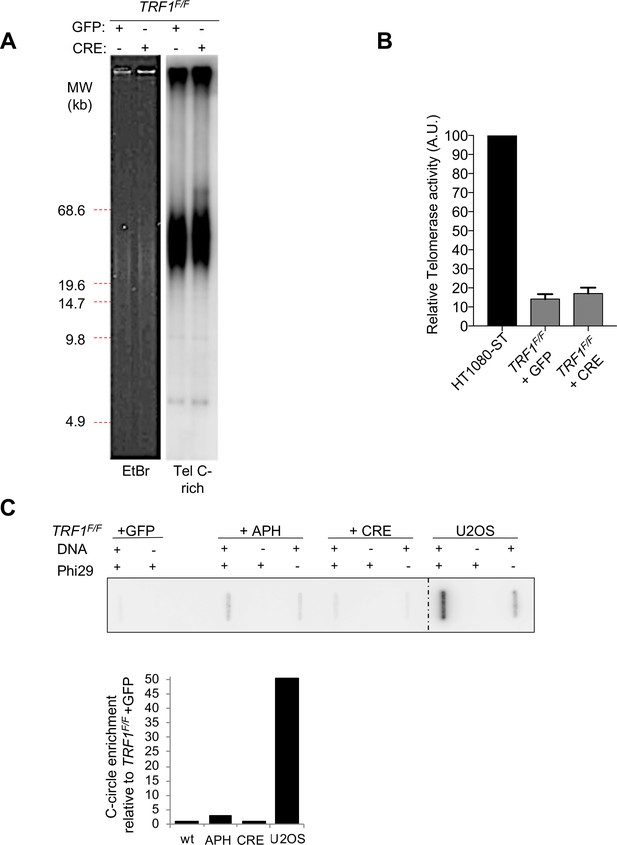
TRF1 deficient MEFs present normal telomere distribution, telomerase activity and no c-circles.
(A) Terminal Restriction Fragments (TRF) blot showing no telomere length heterogeneity upon TRF1 deletion (+CRE, 7 days post-infection) compared to control TRF1F/F +GFP MEFs. The blot was revealed with a DIG-Tel-C-rich probe (right). Ethidium bromide (EtBr) staining (left) is used as loading control. (B) Quantification of telomerase activity levels by TRAP assay showing no changes in telomerase activity after TRF1 deletion in MEFs. Values are normalised to the control HT1080-ST cells (100%) and are represented as mean ± SEM of 4 independent biological replicates. (C) C-circle assay showing no c-circle formation upon TRF1 deletion (+CRE, 7 days post-infection) compared to control TRF1F/F +GFP MEFs. Phi polymerase amplification products were spotted on the membrane and revealed using DIG-TelC probe. TRF1F/F MEFs treated with aphidicolin (APH) are used as negative control for telomere fragility not inducing C-circles, while U2OS, ALT positive cell line, is used as positive control. Source data are provided as a Source Data File.
-
Figure 3—figure supplement 2—source data 1
Telomere length by Southern in TRF1F/F MEFs.
Related to Figure 3—figure supplement 2A.
- https://cdn.elifesciences.org/articles/49817/elife-49817-fig3-figsupp2-data1-v4.xlsx
-
Figure 3—figure supplement 2—source data 2
Telomerase activity by TRAP in TRF1F/F MEFs.
Related to Figure 3—figure supplement 2B.
- https://cdn.elifesciences.org/articles/49817/elife-49817-fig3-figsupp2-data2-v4.xlsx
-
Figure 3—figure supplement 2—source data 3
c-circle amplification assay in TRF1F/F MEFs and U2OS (+ctl).
Related to Figure 3—figure supplement 2C.
- https://cdn.elifesciences.org/articles/49817/elife-49817-fig3-figsupp2-data3-v4.xlsx
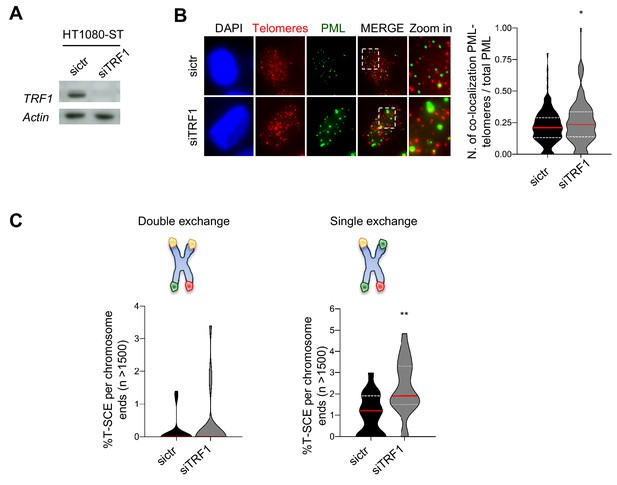
Human TRF1 suppresses APBs formation and BIR.
(A) Western blotting showing expression of TRF1 and Actin (loading control) proteins in human HT1080-ST cell line after depletion of TRF1 with siRNAs (6 days post transfection). sictr is used as negative control for transfection. (B) Representative image of Immunofluorescence showing co-localisation of Telomeres (red) with PML (green) in HT1080-ST nuclei (DAPI) transfected with sictr or siTRF1. Data are represented as number of Telomeres-PML co-localising foci divided by the total number of PML present per nucleus (n = 150 nuclei) and are shown as a violin plot, where the median is underlined in red and quartiles in white. P values, two-tailed student t-test (*, p<0.05). Source data are provided as a Source Data File. (C) Quantification of telomeric T-SCEs in HT1080-ST transfected with sictr or siTRF1. Telomeric exchanges are classified as double exchanges (reciprocal, both chromatids-left graph) and single exchanges (asymmetrical, single chromatid-right graph). Violin plots represent % of T-SCE per chromosome ends (n = at least 1500 events were scored), where the median is underlined in red and quartiles in white. P value, two-tailed student t-test (**, p<0.01).
-
Figure 4—source data 1
KD of TRF1 in HT1080-ST cells.
Related to Figure 4A.
- https://cdn.elifesciences.org/articles/49817/elife-49817-fig4-data1-v4.xlsx
-
Figure 4—source data 2
Quantification of APBs in HT1080-ST TRF1 KD.
Related to Figure 4B.
- https://cdn.elifesciences.org/articles/49817/elife-49817-fig4-data2-v4.xlsx
-
Figure 4—source data 3
T-SCEs quantification in HT1080-ST TRF1 KD.
Related to Figure 4C.
- https://cdn.elifesciences.org/articles/49817/elife-49817-fig4-data3-v4.xlsx
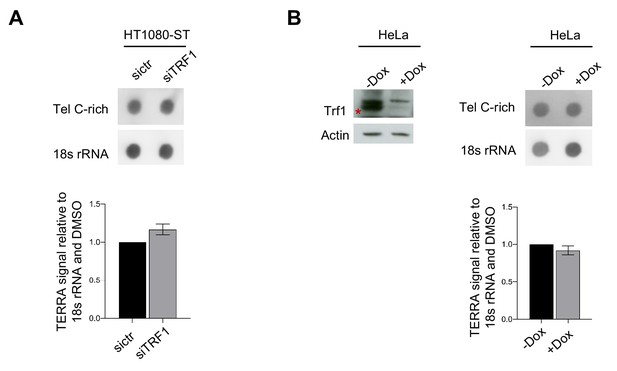
Human TRF1 does not influence TERRAs.
(A) RNA dot-blot analysis in HT1080-ST cells upon sictr or siTRF1 transfection. The blot was revealed with a DIG-Tel-C-rich probe or 18 s rRNA as a control (top panel). Quantification of Data are shown as TERRA signal relative to sictr condition ± SEM of 2 independent biological replicates (bottom panel). P values, two-tailed student t-test. (B) Western blotting (left panel) showing expression of TRF1 and Actin (loading control) proteins in human Hela cell line after deletion of TRF1 (15 days post treatment with doxycycline). -dox is used as negative control. RNA dot-blot analysis in HeLa cells upon deletion of TRF1 (+Dox). The blot was revealed with a DIG-Tel-C-rich probe or 18 s rRNA as a control (top panel). Quantification of Data are shown as TERRA signal relative to -Dox condition ± SEM of 2 independent biological replicates (bottom panel). P values, two-tailed student t-test (*, p<0.05).
-
Figure 4—figure supplement 1—source data 1
Quantification of Telomeric RNA molecules by dot-blot in HT1080-ST TRF1 KD.
Related to Figure 4—figure supplement 1A.
- https://cdn.elifesciences.org/articles/49817/elife-49817-fig4-figsupp1-data1-v4.xlsx
-
Figure 4—figure supplement 1—source data 2
KO efficiency and quantification of Telomeric RNA molecules by dot-blot in Dox inducible HeLa CRISPR/Cas9 system.
Related to Figure 4—figure supplement 1B.
- https://cdn.elifesciences.org/articles/49817/elife-49817-fig4-figsupp1-data2-v4.xlsx
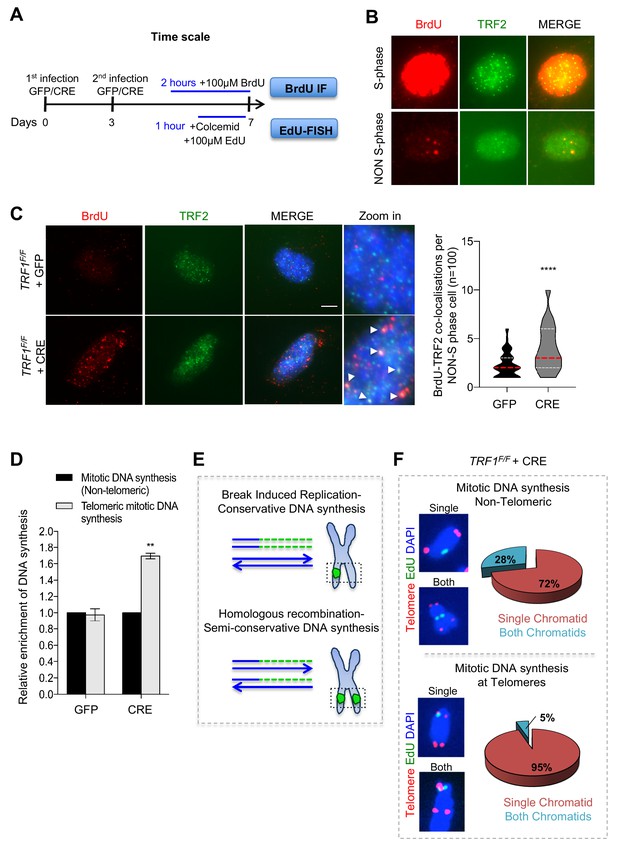
Deletion of TRF1 induces mitotic DNA synthesis at telomeres.
(A) Schematic overview of the experimental timeline. TRF1F/F MEFs cells were infected twice (day 0 and 3) with adenovirus containing either GFP control or CRE to mediate TRF1 deletion. Prior to collection at day 7, cells were treated with either BrdU (100 µM) for 2 hr or EdU (100 µM) + colcemid for 1 hr, to perform respectively BrdU-Immunofluorescence (IF) or EdU-FISH on metaphases. (B) Representative image of BrdU (red) - TRF2 (green) immunofluorescence showing example of cells in S-phase (upper panel) and non-S-phase (bottom panel). (C) Immunofluorescence showing co-localisation of BrdU (red) with TRF2 (green) in TRF1F/F MEFs nuclei (DAPI, blue) treated with GFP and CRE. Scale bar, 5 µm (denaturing conditions). Data are represented in a violin plot as % of cells in non-S-phase showing BrdU-TRF2 co-localising foci (n = 100 nuclei), where the median is underlined in red and quartiles in white, two-tailed student t-test (****, p<0.0001). (D) Quantification of DNA synthesis using the number of EdU-positive intra-chromosomes or telomeres in TRF1F/F cells infected with GFP and CRE relative to the GFP control (n = 50 metaphases). Data are represented as relative enrichment to the GFP control ± SEM of 3 independent biological replicates. P values, two-tailed student t-test (**, p<0.01). (E) Schematic representation of Break Induced Replication (top part) with single EdU foci at a single chromatid and Homologous recombination (bottom part) with EdU foci at both chromatids. (F) Analysis of DNA synthesis in TRF1 deleted cells. Upper panel: Non-telomeric mitotic DNA synthesis. Representative images showing EdU signal (green) in a single chromatid or in both chromatids. Pie chart representing % of chromosomes having EdU signal at a single chromatid or at both chromatids. Bottom panel: Telomeric mitotic DNA synthesis. Representative images showing EdU signal (green) at telomeres (red) at single or both chromatids. Pie chart representing % of chromosomes having EdU signal at telomeres at a single chromatid or both chromatids. Source data are provided as a Source Data File.
-
Figure 5—source data 1
Quantification of BrdU-TRF2 co-localisation in S and non-S nuclei.
Related to Figure 5C.
- https://cdn.elifesciences.org/articles/49817/elife-49817-fig5-data1-v4.xlsx
-
Figure 5—source data 2
Quantification of Mitosis DNA synthesis in TRF1F/F MEFs and at telomeres.
Related to Figure 5D and F.
- https://cdn.elifesciences.org/articles/49817/elife-49817-fig5-data2-v4.xlsx
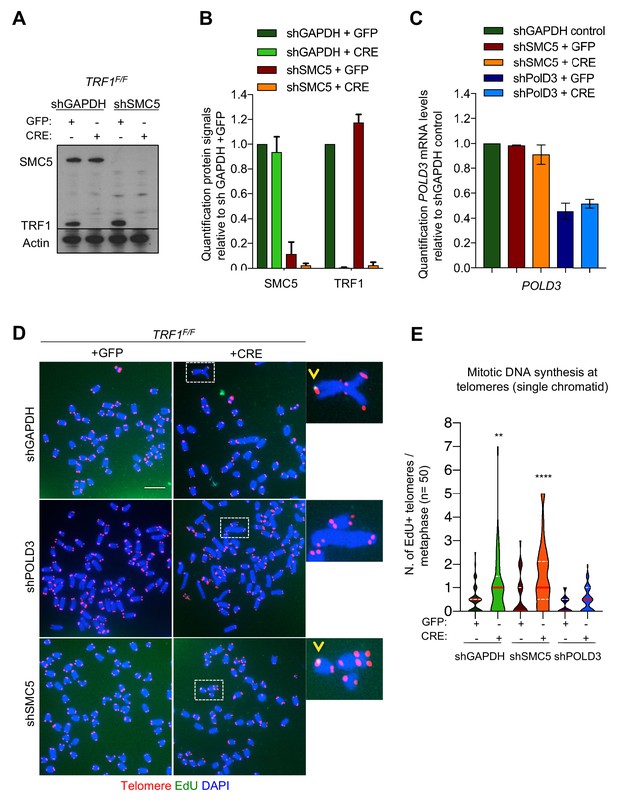
POLD3 but not SMC5 regulates mitotic DNA synthesis at TRF1 deleted telomeres.
(A) Western blotting showing expression of SMC5, TRF1 and Actin (loading control) proteins in TRF1F/F MEFs after infection with GFP or CRE-Adenovirus and deletion of SMC5 by shRNA. shGAPDH is used as negative control. (B) Quantification of the knock-out and knock-down shown in A. Graph shows protein signal quantification relative to shGAPDH in +GFP control cells, data are represented as mean ± SEM of 3 independent biological replicates. (C) Quantification of POLD3 mRNA levels relative to GAPDH control. Data are represented as mean ± SEM of 3 independent biological replicates. (D) Representative images of 6 different genotypes generated in the above description. Metaphases show EdU (green), telomeres labelled with TelPNA-C-rich-Cy3 (red) and chromosomes counterstained with DAPI (blue). Scale bar, 10 µm. (E) Quantification of mitotic DNA synthesis at telomeres (single chromatid) in TRF1F/F MEFs infected with shGAPDH control (GFP or CRE), shSMC5 (GFP or CRE) and shPOLD3 (GFP or CRE). Data are represented (n = 50 metaphases) as number of EdU positive telomeres per metaphase with a violin plot, where the median is underlined in red and quartiles in white. One-way ANOVA multiple comparisons (**, p<0.01; ****, p<0.0001) relative to shGAPDH+GFP sample. Source data are provided as a Source Data File.
-
Figure 6—source data 1
WB and Quantification of SMC5 knock-down efficiency in TRF1F/F MEFs.
Related to Figure 6A and B.
- https://cdn.elifesciences.org/articles/49817/elife-49817-fig6-data1-v4.xlsx
-
Figure 6—source data 2
POLD3 mRNA levels after KD in TRF1F/F MEFs.
Related to Figure 6C.
- https://cdn.elifesciences.org/articles/49817/elife-49817-fig6-data2-v4.xlsx
-
Figure 6—source data 3
Quantification of Mitosis DNA synthesis at telomeres in TRF1F/F MEFs with and without POLD3 and SMC5.
Related to Figure 6D and E.
- https://cdn.elifesciences.org/articles/49817/elife-49817-fig6-data3-v4.xlsx
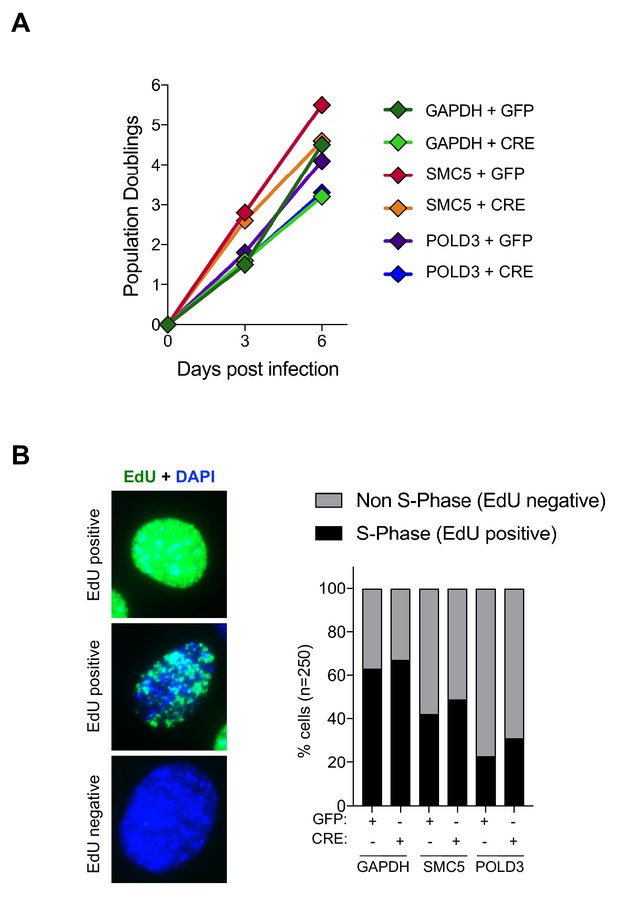
Cell proliferation and EdU incorporation are not affected in TRF1, TRF1-SMC5 and TRF1-POLD3 mutants.
(A) Growth curves showing cell proliferation in TRF1F/F MEFs infected with shGAPDH control (GFP or CRE), shSMC5 (GFP or CRE) and shPOLD3 (GFP or CRE). Population doublings were calculated for each condition. (B) Representative images of IF showing EdU(green) incorporation in MEFs nuclei (DAPI). Quantification of cells (as %) incorporating EdU using IF-staining (n = 250). Cells positive for EdU staining were classified as in S-Phase, while cells negatively stained for EdU were scored as in non-S phase, for the same genetic backgrounds as in A. Source data are provided as a Source Data File.
-
Figure 6—figure supplement 1—source data 1
Population doublings in TRF1F/F MEFs with and without POLD3 and SMC5.
Related to Figure 6—figure supplement 1A.
- https://cdn.elifesciences.org/articles/49817/elife-49817-fig6-figsupp1-data1-v4.xlsx
-
Figure 6—figure supplement 1—source data 2
Number of S- and non-S phase in TRF1F/F MEFs with and without POLD3 and SMC5.
Related to Figure 6—figure supplement 1VB.
- https://cdn.elifesciences.org/articles/49817/elife-49817-fig6-figsupp1-data2-v4.xlsx
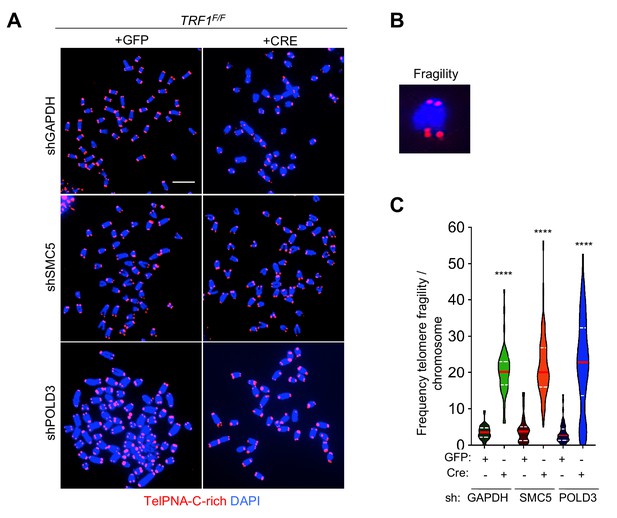
SMC5 and POLD3 are dispensable for TRF1 dependent telomere fragility.
(A) Representative images of metaphases stained with TelPNA-Cy3 probe (red) and DAPI (blue) from TRF1F/F MEFs infected with shGAPDH control (GFP or CRE), shSMC5 (GFP or CRE) and shPOLD3 (GFP or CRE). Scale bar, 10 µm. (B) Enlarged image showing telomere fragility. (C) Quantification of A-B. Data are indicated as % telomere fragility per chromosome. Data from three independent biological replicates are indicated in a violin plot, where the median is underlined in red and quartiles in white. P value, two-tailed student t-test (****, p<0.0001). Source data are provided as a Source Data File.
-
Figure 6—figure supplement 2—source data 1
Fragile telomeres in TRF1F/F MEFs with and without POLD3 and SMC5.
Related to Figure 6—figure supplement 2C.
- https://cdn.elifesciences.org/articles/49817/elife-49817-fig6-figsupp2-data1-v4.xlsx
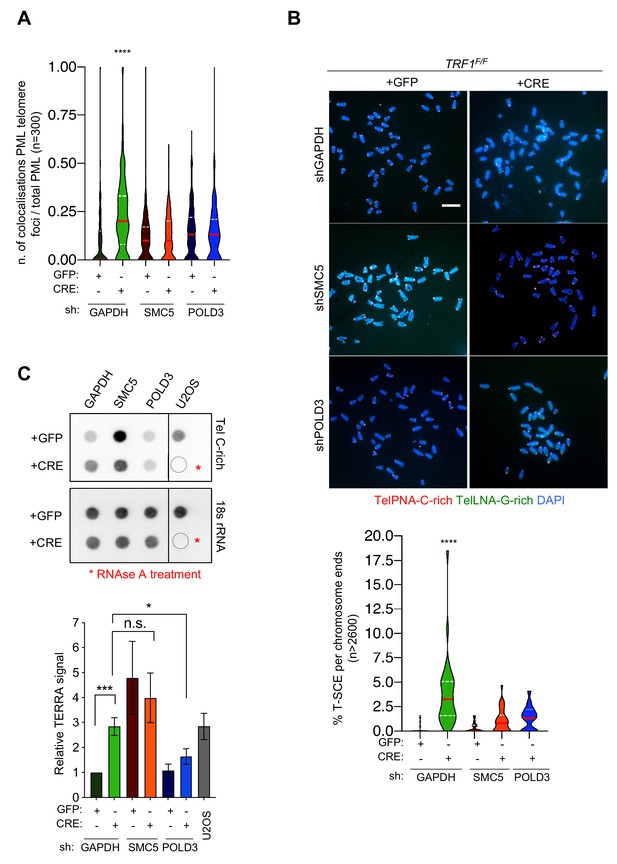
SMC5 and POLD3 are required for induction of recombination at TRF1 deficient telomeres.
(A) APBs formation in TRF1 deleted cells is rescued in double mutants TRF1-SMC5 and TRF1-POLD3. Quantification of APBs formation is represented as number of co-localising PML-telomere foci divided by the total number of PML present per nucleus (n = 300 nuclei analysed) from three independent biological replicates. Data are represented with a violin plot, where the median is underlined in red and quartiles in white. One-way ANOVA multiple comparisons (****, p<0.0001) relative to shGAPDH+GFP sample. (B) Representative images of the chromosome oriented (CO)-FISH assay with denaturation, used to score for telomeric T-SCEs in TRF1F/F MEFs infected with shGAPHH control (GFP or CRE), shSMC5 (GFP or CRE) and shPOLD3 (GFP or CRE). Telomeres are labelled with TelPNA-C-rich-Cy3 (red) and TelLNA-G-rich-FAM (green), while chromosomes are counterstained with DAPI (blue). Scale bar, 10 µm. For quantification T-SCE was considered positive when involved in a reciprocal exchange of telomere signal with its sister chromatid (both telomeres yellow) and for asymmetrical exchanges at single chromatid (one telomere yellow). Data are indicated as % of T-SCE per sister telomere (bottom panel). Data (n = > 2600 chromsome ends) from three independent biological replicates are indicated in a violin plot, where the median is underlined in red and quartiles in white. One-way ANOVA multiple comparisons (****, p<0.0001) relative to shGAPDH+GFP sample. (C) RNA dot blot analysis in TRF1, SMC5, POLD3 single and double mutants. The blot was revealed with a DIG-Tel-C-rich probe or 18 s rRNA as a control. TERRA signals were normalised to 18 s rRNA and GFP control (bottom panel). Data are represented as relative TERRA signal ± SEM of 4 independent biological replicates. P values, two-tailed student t-test (*, p<0.05; ***, p<0.001; n.s. = non significant). Source data are provided as a Source Data File.
-
Figure 7—source data 1
APBs co-localisations quantification in TRF1F/F MEFs with and without POLD3 and SMC5.
Related to Figure 7A.
- https://cdn.elifesciences.org/articles/49817/elife-49817-fig7-data1-v4.xlsx
-
Figure 7—source data 2
T-SCEs quantification in TRF1F/F MEFs with and without POLD3 and SMC5.
Related to Figure 7B.
- https://cdn.elifesciences.org/articles/49817/elife-49817-fig7-data2-v4.xlsx
-
Figure 7—source data 3
Quantification of Telomeric RNA molecules by dot-blot in TRF1F/F MEFs with and without POLD3 and SMC5.
Related to Figure 7C.
- https://cdn.elifesciences.org/articles/49817/elife-49817-fig7-data3-v4.xlsx
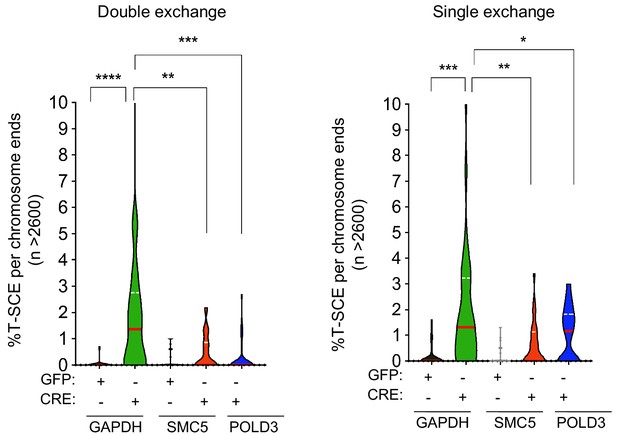
SMC5 and POLD3 are required for TRF1 dependent telomere recombination.
Quantification of telomeric T-SCEs in TRF1F/F MEFs infected with shGAPDH control (GFP or CRE), shSMC5 (GFP or CRE) and shPOLD3 (GFP or CRE). Telomeric exchanges are classified as double exchanges (reciprocal, both chromatids-left graph) and single exchanges (asymmetrical, single chromatid-right graph). Violin plots represent % of T-SCE per chromosome ends (n = at least 2500 events were scored), where the median is underlined in red and quartiles in white. P value, two-tailed student t-test (*, p<0.05; **, p<0.01; ***, p<0.001; ****, p<0.0001).
-
Figure 7—figure supplement 1—source data 1
T-SCEs quantification in TRF1F/F MEFs with and without POLD3 and SMC5.
Related to Figure 7—figure supplement 1A. Accession code for the proteomic data will be made available before publication on public repository PRIDE.
- https://cdn.elifesciences.org/articles/49817/elife-49817-fig7-figsupp1-data1-v4.xlsx
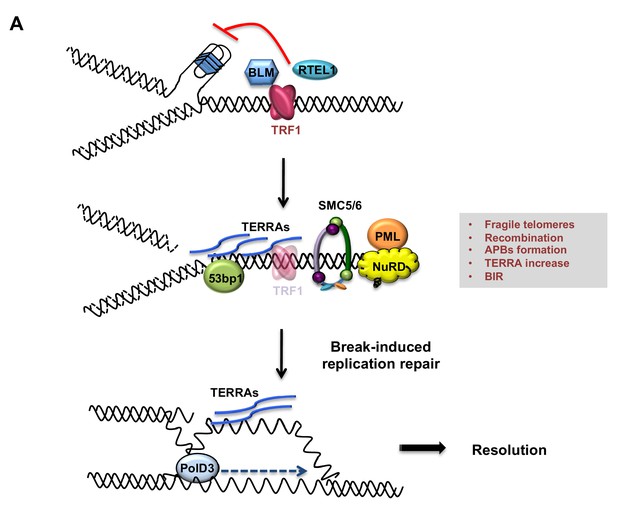
Model describing TRF1 as a negative regulator of telomeric transcription (TERRAs), APBs formation, telomeric recombination via PolD3-BIR dependent pathway.
Replicative stress induced by TRF1 deletion alters the chromatin status of these telomeres. Recruitment of chromatin remodellers/HR factors, TERRA accumulation and telomere fragility are observed. The SMC5/6 complex and polymerase POLD3 are among the factors recruited at replicative-stressed telomeres, representing the key players for APBs formation and telomere recombination, particularly BIR-mechanism. We propose that increased TERRAs molecules at telomeres could lead to increased R-loops, which are bypassed by POLD3 dependent BIR to resolve fork progression hindrance.
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Cell line (Homo-sapiens) | HT1080-ST fibrosarcoma cell line | kind gift from J Lingner Cristofari and Lingner, 2006 | ||
| Cell line (Homo-sapiens) | TRF1 CRISPR/Cas9 HeLa cell line | kind gift from Z Songyang Kim et al., 2017 | Doxycycline-inducible | |
| Cell line (M. musculus) | TRF1F/F Mouse Embryonic Fibroblasts | Established in Boulton Lab (Crick Institute) Sfeir et al., 2009 | RRID:CVCL_UE12 Primary and SV40 immortalised | |
| Transfected construct (human) - in HT1080-ST | TERF1 siRNA SMARTpool: ON-TARGETplus | Dharmacon/Thermo Fisher Scientific | #L-010542-02-0020 | 100 nM |
| Transfected construct (human) - in HT1080-ST | ctl siRNA ON-TARGETplus Non-targeting Pool | Dharmacon | #D-001810-10-20 | 100 nM |
| Other | Nucleofector II/2b Device | Lonza | #LO AAB-1001 | programme X-001. |
| Commercial assay, kit | Nucleofector kit T | Lonza | #VVCA-1002 | |
| Transfected construct (Mouse) | pLKO.1-puromycin lentiviral vectors shRNA for mouse SMC5 | Sigma | sequence: CCCATAATGCTCACGATTAAT | in MEFs |
| Transfected construct (Mouse) | pLKO.1-puromycin lentiviral vectors shRNA for mouse POLD3 | Sigma | Sequence: GCATATACTCATGTGTGGTTT | in MEFs |
| Transfected construct (Mouse) | pLKO.1-puromycin lentiviral vectors GAPDH | Open biosystems | Sequence: CTCATTTCCTGGTATGACA | in MEFs |
| Software, algorithm | PRISM eight software, Statistical analysis | GraphPad | ||
| Chemical compound, drug | DAPI stain | Invitrogen | D1306 | (1 µg/mL) |
| Antibody | IgG, Rabbit polyclonal | Abcam | ab37415 | For ChIP 2 μg of antibody |
| Antibody | TRF2, Rabbit polyclonal | Novus | NB110−57130/B2 | For ChIP 5 μg of antibody |
| Antibody | BRCA1, Rabbit polyclonal | Novus | NBP1-45410 | For ChIP 5 μg of antibody |
| Antibody | BAZ1b, Rabbit polyclonal | Cell Signaling | 2152S | For ChIP 5 μg of antibody |
| Antibody | TR4, Mouse monoclonal | R and D biosystems | pp-H0107B-00 | For ChIP 5 μg of antibody |
| Antibody | P66a, Rabbit polyclonal | Novus | NBP1-87359 | For ChIP 5 μg of antibody |
| Antibody | MTA1, Rabbit polyclonal | Abcam | ab71153 | For ChIP 5 μg of antibody |
| Antibody | CHD4, Rabbit polyclonal | Novus | NB100-57521 | For ChIP 5 μg of antibody |
| Antibody | ZNF827, Mouse monoclonal | Santa Cruz | sc514943 | For ChIP 5 μg of antibody |
| Antibody | IgG, Rabbit polyclonal | Abcam | ab37415 | For IF (1:1000) |
| Antibody | TRF2, Rabbit polyclonal | Novus | NB110−57130/B2 | For WB (1:1000) and IF (1:250) |
| Antibody | TRF2, Rabbit polyclonal | Gift from T de Lange | Ref: 1254 | For WB (1:1000) and IF (1:250) |
| Antibody | TRF1, Rabbit polyclonal | Gift from T de Lange | Ref: 1449 | For WB (1:1000) and IF (1:250) |
| Antibody | PML, Rabbit polyclonal | Gift from Paul Freemont | / | For IF (1:250) |
| Antibody | SMC5, Rabbit polyclonal | Gift from Jo Murray | / | For WB (1:1000) and IF (1:250) |
| Antibody | Beta-actin, Mouse monoclonal | Abcam | ab8226 | For WB (1:5000) |
| Antibody | BrdU, Mouse monoclonal | MBL | MI-11–3 | For IF (1:500) |
| Antibody | Anti-rabbit Alexa 488 antibody, Donkey polyclonal | Thermo, | A21206 | For IF (1:2000) |
| Antibody | Anti-mouse Ig-HRP, Goat polyclonal | DAKO | P0447 | For WB (1:5000) |
| Antibody | Anti-Rabbit Ig-HRP, Pig polyclonal | DAKO | P0217 | For WB (1:10000) |





