Homoplasy in the evolution of modern human-like joint proportions in Australopithecus afarensis
Abstract
The evolution of bipedalism and reduced reliance on arboreality in hominins resulted in larger lower limb joints relative to the joints of the upper limb. The pattern and timing of this transition, however, remains unresolved. Here, we find the limb joint proportions of Australopithecus afarensis, Homo erectus, and Homo naledi to resemble those of modern humans, whereas those of A. africanus, Australopithecus sediba, Paranthropus robustus, Paranthropus boisei, Homo habilis, and Homo floresiensis are more ape-like. The homology of limb joint proportions in A. afarensis and modern humans can only be explained by a series of evolutionary reversals irrespective of differing phylogenetic hypotheses. Thus, the independent evolution of modern human-like limb joint proportions in A. afarensis is a more parsimonious explanation. Overall, these results support an emerging perspective in hominin paleobiology that A. afarensis was the most terrestrially adapted australopith despite the importance of arboreality throughout much of early hominin evolution.
Introduction
Among extant hominoids, modern humans (Homo sapiens; hereafter, ‘humans’) are the only habitually bipedal species. Adaptation to upright walking and running in humans is evidenced by the presence of a host of postcranial morphologies functionally related to saving mechanical and metabolic energy (Lovejoy, 1988; Bramble and Lieberman, 2004; Pontzer, 2017). These include relatively long legs, arched feet (Venkadesan et al., 2020), and adaptations to protect the joints of the lower limbs from excessive stress by increasing their surface areas relative to the mass of the body (Ruff, 1988; Jungers, 1988; Lovejoy, 2005). These morphological traits are most strongly expressed in recent modern humans, which are nearly exclusively terrestrial in their locomotor adaptation. In contrast, the body plan of extant, nonhuman apes (hereafter, simply ‘apes’) reflects an adaptation to orthogrady and suspension (Keith, 1923; Gebo, 1996; Williams and Russo, 2015) with relatively long arms and large upper limb joints (Ruff, 1988), elongated, curved phalanges (Deane and Begun, 2008), and other morphological features suitable for arboreal behaviors (Gebo, 1996). Although chimpanzees, bonobos, and gorillas possess adaptations to terrestrial quadrupedalism (Gebo, 1996), including their knuckle-walking hand posture and heel-strike plantigrade foot posture (Gebo, 1992; Prang, 2019), they retain traits linked to an ancestry characterized by vertical climbing and suspension in some form (Gebo, 1996).
The relatively larger upper limb joints of apes compared to humans reflect disparate joint loading regimes associated with forms of quadrupedalism, climbing, and suspension (Gebo, 1996). Additionally, the relatively larger surface areas of the convex side of conarticular joints may contribute to increased range of motion, providing benefits to the arboreal locomotor performance of apes (Ruff, 1988; Godfrey et al., 1991; Hammond, 2014; Prang, 2016). Therefore, the relative size of postcranial joints and the relationship between the joints of the upper and lower limbs are important correlates of positional and locomotor behavior among hominoids (Ruff, 1988; Jungers, 1988; Godfrey et al., 1995; McHenry, 1992; McHenry and Berger, 1998; Green et al., 2007; Haeusler and McHenry, 2007).
The timing and pattern of the complicated, nonlinear evolutionary loss of adaptations to arboreality and the transition to a form of nearly exclusive terrestrial bipedalism among hominins has been debated for decades (Stern, 2000; Ward, 2002). The study of limb joint proportions initially focused on the preserved partial skeletons A.L. 288-1 (Australopithecus afarensis) and StW 431 (Australopithecus africanus) (McHenry and Berger, 1998; Green et al., 2007), along with OH 62 and KNM-ER 3735 (Homo habilis; Haeusler and McHenry, 2007; Johanson et al., 1987; Leakey et al., 1987). Previous studies have shown that the geologically younger A. africanus possessed relatively large upper limb joints and metaphyseal dimensions in comparison to A. afarensis (McHenry and Berger, 1998; Green et al., 2007). The OH 62 and KNM-ER 3735 partial skeletons are more fragmentary, but morphological comparisons of external morphology (Haeusler and McHenry, 2007; Hartwig-Scherer and Martin, 1991) and cross-sectional geometry (Ruff, 2009) suggest that the upper limbs of H. habilis bore similarities to extant chimpanzees and gorillas, implying greater reliance on forelimb-dominated behaviors, and may show a similar pattern to that observed in A. africanus (McHenry and Berger, 1998; Green et al., 2007; Haeusler and McHenry, 2007). The observed pattern of joint size proportions among extant hominoids implies that the relatively larger lower limb joints of A. afarensis are a reflection of increased terrestriality compared to A. africanus.
Cladistic analyses of hominin phylogeny based on craniodental characters consistently position A. africanus as more closely related to Homo than is A. afarensis (Dembo et al., 2016; Strait et al., 2015). Therefore, either (1) A. afarensis and H. sapiens independently evolved relatively larger lower limb joints (i.e., their similarities are homoplastic), (2) A. africanus and H. habilis evolved more ape-like joint proportions from an ancestor with more human-like limb proportions (i.e., the similarities between A. africanus, H. habilis, and apes are homoplastic), or (3) A. afarensis is more closely related to Homo than are A. africanus and H. habilis (Figure 1). Limited taxonomic sampling of fossil hominins in previous studies has rendered these competing scenarios exceedingly difficult to differentiate. Over the past few decades, however, the recovery of new fossil hominin partial skeletons preserving both upper and lower limb joints has provided an expanded sample that can be used to evaluate these hypotheses more rigorously (Table 1). Here, we re-examine the upper and lower limb joint proportions of multiple species of Australopithecus, Paranthropus, and Homo to evaluate these long-standing alternative hypotheses for patterns of postcranial evolution in hominins.
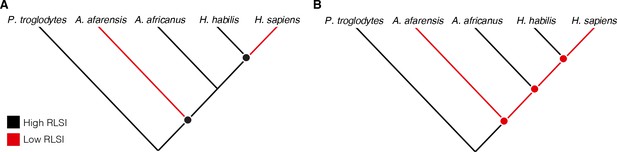
Alternative hypotheses to explain the pattern of limb joint proportions observed in the human fossil record.
Previous work interpreted the human-like ratio of upper to lower limb joint size (relative limb size index [RLSI]) in Australopithecus afarensis to indicate either (A) homoplasy between A. afarensis and Homo sapiens or (B) evolutionary reversals to a more ape-like body form in A. africanus and H. habilis.
Fossil hominin and extant hominoid measurements.
| Specimen | Taxon | G | H | B | U | R | F | Sub | A | T | Sac |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Homo sapiens | 29.5 ± 2.7 (N = 67) | 42.3 ± 3.5 (N = 67) | 59.1 ± 4.7 (N = 52) | 21.8 ± 1.9 (N = 51) | 21.7 ± 2.1 (N = 51) | 44.5 ± 3.5 (N = 67) | 28.6 ± 2.1 (N = 52) | 51.2 ± 3.5 (N = 67) | 28.2 ± 2.0 (N = 66) | 38.6 ± 3.1 (N = 67) | |
| Pan | 26.6 ± 2.4 (N = 113) | 38.2 ± 3.1 (N = 113) | 62.5 ± 5.5 (N = 95) | 22.5 ± 2.8 (N = 95) | 24.5 ± 1.8 (N = 94) | 32.8 ± 2.5 (N = 120) | 25.0 ± 2.0 (N = 98) | 38.6 ± 3.3 (N = 116) | 18.1 ± 34.1 (N = 116) | 28.4 ± 3.9 (N = 109) | |
| Gorilla | 39.2 ± 5.5 (N = 119) | 54.9 ± 7.3 (N = 122) | 93.0 ± 13.0 (N = 94) | 33.4 ± 5.7 (N = 89) | 31.7 ± 4.4 (N = 91) | 46.6 ± 5.9 (N = 125) | 35.7 ± 4.9 (N = 93) | 53.1 ± 6.9 (N = 114) | 24.9 ± 5.6 (N = 108) | 37.7 ± 5.8 (N = 102) | |
| Pongo | 29.2 ± 3.6 (N = 47) | 40.0 ± 4.8 (N = 49) | 63.9 ± 7.1 (N = 45) | 22.1 ± 3.2 (N = 46) | 22.8 ± 2.8 (N = 46) | 32.9 ± 4.0 (N = 49) | 20.8 ± 2.6 (N = 45) | 39.0 ± 4.7 (N = 49) | 18.0 ± 2.7 (N = 46) | 28.0 ± 4.1 (N = 43) | |
| Hylobatids | 13.2 ± 1.7 (N = 62) | 18.5 ± 2.3 (N = 66) | 28.0 ± 3.1 (N = 66) | 11.0 ± 1.5 (N = 66) | 12.6 ± 1.5 (N = 69) | 16.4 ± 2.1 (N = 65) | 11.0 ± 1.5 (N = 65) | 20.6 ± 3.0 (N = 66) | 7.5 ± 1.0 (N = 59) | 14.7 ± 2.3 (N = 58) | |
| A.L. 288-1 | A. afarensis | 21.6 | 28.9 | 41.1 | 16.1 | 15.1 | 28.6 | 20.8 | 37.0 | 18.0 | 25.3 |
| KSD-VP-1/1 | A. afarensis | 30.1 | 58.8 | 49.0 | 32.4 | ||||||
| DIK-1-1 | A. afarensis | 13.5 | 13.1 | ||||||||
| StW 573 | A. prometheus (?); A. africanus | 25.9 | 31.3 | 54.0 | 24.3 | 21.9 | 35.2 | 24.5 | 43.0 | 18.0 | |
| StW 431 | A. africanus | 59.0 | 25.7 | 21.9 | 45.0 | 27.5 | |||||
| MH1 | A. sediba | 57.0 | 18.9 | 33.0 | 23.2 | 22.0 | |||||
| MH2 | A. sediba | 24.6 | 30.1 | 52.4 | 17.4 | 18.8 | 32.7 | 18.1 | 23.6 | ||
| BOU-VP-12/1 | A. garhi(?) | 21.4 | 23.7 | ||||||||
| TM 1517 | P. robustus | 54.0 | 22.0 | 18.9 | |||||||
| OH 80 | P. boisei | 26.3 | 26.5 | ||||||||
| KNM-ER 1500 | P. boisei | 21.4 | 20.2 | 24.2 | 19.2* | ||||||
| KNM-ER 1503/1504 | P. boisei | 57.0 | 30.6 | 22.2 | |||||||
| KNM-ER 3735 | H. habilis | 55.0 | 20.0 | 25.3 | |||||||
| KNM-WT 15000 | H. erectus | 27.6 | 31.6 | 55.0 | 19.0 | 46.0 | 28.8 | 25.0* | 33.6 | ||
| LES 1 | H. naledi | 33.2 | 16.1 | 36.0 | 24.2 | 24.5 | |||||
| LB 1 | H. floresiensis | 19.5 | 31.0 | 22.1 | 36.0 | 19.5 |
-
*Estimated from tibial plafond width.
-
G: glenoid size (geomean of SI height and AP width); H: humeral head diameter (geomean of SI height and AP width); B: humeral biepicondylar breadth; U: ulna olecranon width; R: radial head diameter; F: femoral head diameter; Sub: femoral subtrochanteric width (geomean of ML width and AP breadth); A: acetabulum height; T: talar mediolateral width; Sac: sacral size (geomean of ML width and AP breadth). ±1 standard deviation is given with sample size (N=#).
Results
We quantified limb joint proportions per individual using the relative limb size index (RLSI) (Green et al., 2007). The RLSI is the logged ratio of geometric means calculated from upper (forelimb) and lower (hindlimb) limb measurements and quantifies whether a given specimen has relatively larger forelimb or hindlimb joints (Green et al., 2007). We calculated a series of RLSIs to accommodate the differential preservation of postcranial elements among the 16 hominin partial skeletons sampled here. When the full upper to lower limb dataset is used, there is clear separation between humans, with their proportionally larger lower limbs, and modern apes, with their proportionately larger upper limbs, with no overlap. Importantly, there remains clear separation between humans and great apes in cases where truncated datasets were used to quantify the limb joint proportions of less complete hominin skeletons. The ape data, however, do not always accord with degree of arboreality (hylobatid > Pongo > Pan > Gorilla).
5 of the 16 partial hominin skeletons are human-like in their limb joint proportions (Figure 2, Figure 2—figure supplements 1–26). The RLSI of A.L. 288-1 (Lucy) is far outside the ape range and falls squarely within the range of modern humans (Figure 2, Figure 2—figure supplement 1). Likewise, the larger, presumed male A. afarensis partial skeleton KSD-VP-1/1 is positioned within the human range, though it overlaps with the low end of the hylobatid distribution (Figure 2—figure supplement 2). The infant partial skeleton of A. afarensis (DIK-1-1), as well as Lucy, has a human-like glenoid:talus ratio (Figure 2—figure supplement 3). KNM-WT 15000 (Homo erectus) has even larger relative lower limb joint proportions than the humans sampled in this study and is well outside the ape range (Figure 2—figure supplement 4). LES 1 (Homo naledi) falls within the human interquartile range, outside any modern ape distribution (Figure 2—figure supplement 5).
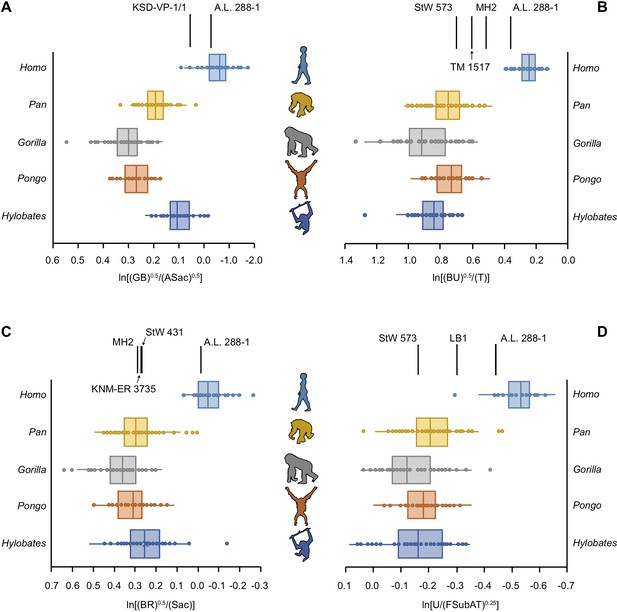
Relative limb size index (RLSI) in modern apes, humans, and fossil hominins.
Notice that A.L. 288-1 (Lucy) falls within the modern human distribution for RLSI no matter which combination of upper to lower limb joint proportions is examined (A–D). (A) Human-like upper to lower limb joint proportions remain human-like on the basis of preserved elements in a second partial skeleton of A. afarensis, KSD-VP-1/1. However, all other partial skeletons of Australopithecus (A–C), Paranthropus (B) and early Homo (C) are more ape-like. A high, ape-like RLSI is present even in the late Pleistocene hominin H. floresiensis (D).
-
Figure 2—source data 1
Raw measurements on extant primate skeletons.
- https://cdn.elifesciences.org/articles/65897/elife-65897-fig2-data2-v1.xlsx
All of the other hominin skeletons studied fall outside the human range, indicating that they are more ape-like in their joint proportions (Figure 2). StW 431 (A. africanus) has limb proportions positioned within the hylobatid interquartile range and within the distributions of Pan, Gorilla, and Pongo (Figure 2—figure supplement 6). MH1 (juvenile Australopithecus sediba) falls within the interquartile range of hylobatids and Pan and within the ranges of Gorilla and Pongo (Figure 2—figure supplement 7). MH2 (adult A. sediba) occupies the space between great apes and humans, positioned only within the range of hylobatids (Figure 2—figure supplement 8). StW 573 is similar to MH2 in having relatively larger upper limb joints than modern humans but smaller than extant apes, positioned only near a hylobatid outlier (Figure 2—figure supplement 9).
Partial skeletons attributed to Paranthropus all possess ape-like joint proportions. TM 1517 (Paranthropus robustus) and KNM-ER 1500 (Paranthropus boisei) fall within the ranges of all four extant apes (Figure 2—figure supplements 10 and 11). OH 80 (P. boisei) falls within the interquartile range of Pan and the range of Gorilla and hylobatids (Figure 2—figure supplement 12). Associated fossils KNM-ER 1503/1504 (tentatively attributed to P. boisei) have joint proportions within the interquartile range of Pan, Gorilla, and hylobatids (Figure 2—figure supplement 13).
BOU-VP-12/1 (Australopithecus cf. garhi) falls squarely within the Gorilla interquartile range and within the lower range of Pan (Figure 2—figure supplement 14). There is a single human outlier overlapping with the limb joint proportions of BOU-VP-12/1.
KNM-ER 3735 (H. habilis) has joint proportions in the hylobatid interquartile range and within the ranges of all extant great apes (Figure 2—figure supplement 15). The joint proportions of LB 1 (Homo floresiensis) fall within the ranges for all of the apes, though there is a single human outlier similar in joint proportions to LB 1 (Figure 2—figure supplement 16).
Parsimony reconstructions suggest that the human-like limb joint proportions in A. afarensis and modern humans are homoplastic, regardless of the phylogenetic hypothesis used (Figure 3). The only phylogenetic hypothesis that does not require either homoplasy or multiple reversals is one in which A. afarensis is more derived than H. habilis, H. floresiensis, and all other species of Australopithecus and Paranthropus examined in this study. Given that an A. afarensis-later Homo clade (to the exclusion of early Homo) has not been supported by any phylogenetic analysis, we consider this last scenario unlikely in the extreme.
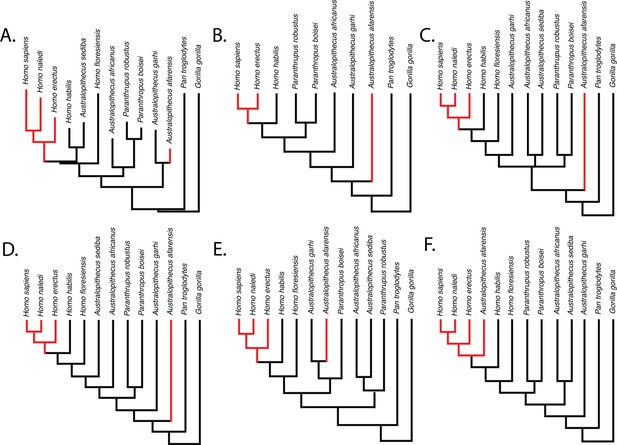
Relative limb size index (RLSI; high in black; low in red) for the taxa examined in this study.
The phylogenies in (A) and (B) are from Dembo et al., 2016 (A) and Mongle et al., 2019 (B). The phylogenies in (C–E) presented above are informed by various hypotheses about the relationships of Australopithecus and Paranthropus taxa that have been published but not recovered in formal phylogenetic analyses. These include the hypothesis that Australopithecus garhi is a unique ancestor of Homo (C; Asfaw et al., 1999), that Australopithecus sediba is a unique ancestor of Homo (D; Berger et al., 2010; Irish et al., 2013), and the hypothesis that Paranthropus is actually polyphyletic (E; topology based on hypothetical tree presented in Wood and Schroer, 2017). A hypothetical phylogeny in which Australopithecus afarensis is more derived than two species of Homo as well as all other Australopithecus and Paranthropus species (such as shown in F) would need to be correct for the pattern of RLSI in hominins to be best explained as anything other than homoplasy between A. afarensis and some later Pleistocene Homo.
Discussion
Apes have relatively larger upper limb than lower limb joints as reflected by their higher RLSI than modern humans (Green et al., 2007). With musculoskeletal anatomies adapted for climbing and suspension, apes possess larger upper limb muscles and joints with greater surface areas, which has the effect of limiting excessive stresses and strains arising from large joint reaction forces. Enlarged upper limb joint surface areas in apes may also contribute to increased ranges of motion (e.g., at the glenohumeral joint). In contrast, humans are characterized by relatively larger lower limb joints, which act to reduce stresses and strains on the joints and nonrenewable cartilage of the hip, knee, and ankle arising from repetitive high-magnitude ground and joint reaction forces during heel-striking bipedal walking and running. This pattern accords with expectations based on the posture and locomotion of apes and humans. Apes possess heavily built upper limbs associated with orthograde climbing and suspension, whereas modern humans have robust lower limbs adapted to terrestrial bipedalism. This morphological pattern provides a framework for interpreting the functional and evolutionary implications of joint proportions in fossil hominins.
It is noteworthy, however, that the ape RLSI did not always align with degree of arboreality (hylobatid > Pongo > Pan > Gorilla). As reported elsewhere (Gordon et al., 2020), the preserved anatomical elements used to calculate RLSI can impact where a taxon is positioned along a locomotor continuum. The relatively narrow great ape sacrum, hypothesized to facilitate entrapment of the lumbar vertebrae and stiffen the lower back during climbing, further increases the RLSI of Pan, Gorilla, and Pongo relative to the hylobatids (Figure 4—figure supplements 1–4). Additionally, while RLSI calculations that included the radial head and ulnar trochlear width separate the ape species by locomotor mode, those that include the glenoid size and the biepicondylar breadth do not. In fact, a post hoc examination of limb joint scaling found that while great apes and humans exhibit isometric scaling of the glenoid and humeral biepicondylar breadth relative to femoral head diameter, the hylobatids scale with negative allometry (glenoid m = 0.75; biepicondylar breadth m = 0.76). It is likely that the differing sizes of these apes and the functional demands on the limb joints in arboreal apes across this size range are driving some of the unexpected RLSI results reported here.
Our study provides a fresh perspective on alternative hypotheses for the evolution of limb joint proportions introduced by previous workers (McHenry and Berger, 1998; Green et al., 2007; Haeusler and McHenry, 2007). The reconstruction of patterns of hominin evolution relies on phylogeny, and, since the early adoption of cladistics, no quantitative analysis of hominin phylogeny has recovered a sister taxon relationship between A. afarensis and Homo (Dembo et al., 2016; Strait et al., 2015). The recovery of purported Homo fossils significantly predating the appearance of A. africanus and A. sediba may falsify hypotheses of exclusive ancestry and descent (Du and Alemseged, 2019). However, the consistent placement of A. africanus and A. sediba near Homo implies that they share a more recent common ancestor than do Homo and A. afarensis, despite their temporal and geographic distance (Dembo et al., 2016; Berger et al., 2010; Irish et al., 2013; Pickering et al., 2011). The inclusion of Paranthropus and early Homo fossils here helps alleviate the evolutionary implications of uncertainty surrounding the phylogenetic positions of Australopithecus species. The homology of a low RLSI in A. afarensis and later Homo can only be explained by an increasingly large number of evolutionary reversals. The independent evolution of similar limb joint proportions in A. afarensis and later Homo is a more parsimonious interpretation of the data.
There exists one additional piece of evidence that supports our interpretation that the low RLSI of A. afarensis and modern humans is homoplastic. Interestingly, the low RLSI of A. afarensis was achieved with a different morphological pattern compared to H. erectus. We found that H. erectus possessed a relatively smaller sacral body (like A. africanus and A. sediba) but a large femoral head relative to the upper limb than do modern humans; however, A. afarensis possessed a relatively small femoral head and large sacral body (Figure 4). This finding may further imply parallel evolution in limb joint proportions between A. afarensis and H. sapiens. The relatively small sacral body of the female H. erectus sacrum from Gona (Simpson et al., 2008, personal observation) demonstrates that these results are not the result of the juvenile status of KNM-WT 15000.
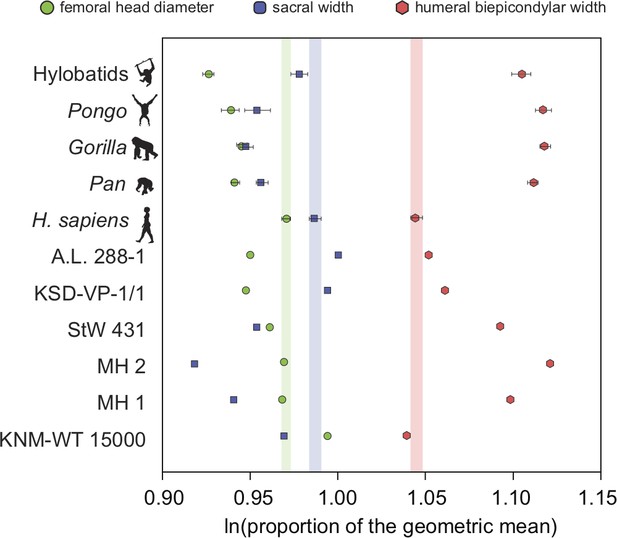
Additional evidence for homoplasy in relative limb size index (RLSI) between A.afarensis and H. sapiens is presented here.
Only femoral head diameter, sacral width, and humeral biepicondylar width are considered in this analysis and all extant apes and hominin fossils are shown relative to the modern human condition (vertical-colored stripes). Horizontal bars are 95% confidence intervals (with those of human highlighted in vertical colored bars). Note that as in apes, A. africanus (StW 431) and A. sediba (MH1 and MH2) have a relatively large humeral biepicondylar width and relatively small sacrum. A. afarensis (A.L. 288-1 and KSD-VP-1/1) has a slightly larger biepicondylar breadth and sacral width with a slightly smaller femoral head relative to modern humans, though as already demonstrated, the overall RLSI is human-like. However, while H. erectus (KNM-WT 15000) also possesses a human-like RLSI, it is accomplished in a different anatomical manner. Notice that the colored dots (blue and green) are reversed in H. erectus relative to both A. afarensis and H. sapiens, meaning that in H. erectus the sacrum is smaller than expected (as in other australopiths) and the femoral head larger than expected. The BSN49/P27 H. erectus pelvis possesses a similarly small sacrum, indicating that this result is not solely a result of the juvenile status of KNM-WT 15000.
The morphology and functional anatomy of the axial skeleton, pelvis, and lower limb display unambiguous evidence for bipedal posture and locomotion in Australopithecus and later hominins. Furthermore, the presence of traits potentially signifying the importance of arboreality among fossil hominins does not necessarily imply reduced bipedal competency. However, the distributions of RLSI data (Figure 2—figure supplements 17–26), along with observations of other regional anatomies, imply differences among hominins in their adaptation to terrestrial, heel-striking bipedality. A. afarensis has relatively larger lower limb joints than any other early hominin currently known and possesses features of the foot and ankle that imply bipedal performance capabilities exceeding those of later early hominins. These traits include a more robust calcaneal tuber, a flatter subtalar joint, and a more plantarly oriented fourth metatarsal diaphysis and talonavicular joint (reviewed in DeSilva et al., 2019). The available morphological evidence suggests that, compared to other Plio-Pleistocene hominins, A. afarensis was better able to withstand the stresses and strains induced by the repetitive loading of the lower limb in frequent terrestrial bipedalism.
Over the past three decades, significant emphasis has been placed on the retention of ape-like characters in Australopithecus and Paranthropus since they could have been maintained through stabilizing selection if arboreality was a significant part of their positional repertoires (Stern, 2000). However, many researchers have repeatedly noted the difficulty of distinguishing the effects of stabilizing selection from those of evolutionary inertia (or ‘lag’). This critique is rooted in a maximum likelihood, character-based cladistic framework, which implicitly excludes information about the evolutionary process (e.g., evolutionary rates as a function of time). In other words, primitive retentions have the same meaning across varying temporal ranges in a traditional cladistic framework. The presence of presumed primitive, ape-like features in late Australopithecus and early Homo c. ~2 Ma implies that evolutionary processes, whether neutral (i.e., genetic drift) or non-neutral (i.e., directional selection), had not yet substantially modified them over a 4- to 5 million-year period given current estimates of the Pan-Homo divergence date. Therefore, in our view, primitive retentions in Australopithecus, Paranthropus, and Homo can be meaningful when interpreted within the context of the evolutionary process.
Regardless of assumptions underlying evolutionary processes, primitive retentions in late Australopithecus and early Homo occur alongside indirect evidence for arboreal activity in their trabecular bone density patterns and long bone diaphyseal properties. A recent study of trabecular bone density of the non-pollical metacarpal heads of A. sediba showed a close morphometric affinity with extant orangutans, despite having human-like hand proportions (Dunmore et al., 2020). The external structure and internal trabecular morphology of the A. sediba hand are consistent with the use of forceful metacarpophalangeal joint flexion, which is a requisite of forelimb-dominated, below-branch locomotion (Dunmore et al., 2020). The purportedly more ape-like limb joint and length proportions of the OH 62 partial skeleton are supported by a more chimpanzee-like humeral cross-sectional geometry, implying that the H. habilis upper limb was heavily built (Ruff, 2009). The femoral head trabecular bone density pattern of StW 311 from Sterkfontein attributed either to P. robustus or Homo sp. implies the more habitual use of flexed hip postures, which occurs during climbing (Georgiou et al., 2020).
In light of the congruence between the functional interpretations derived from the external and internal morphology of hominin postcranial fossils, we consider the limb joint proportions data presented here, and in previous studies, to be a reliable indicator of adaptation to arboreal locomotion. The relatively larger hindlimb joints of A. afarensis and later Homo are consistent with a more pronounced terrestrial component of their positional repertoires, whereas the relatively larger upper limb joints of most Australopithecus, Paranthropus, and early Homo individuals indicate a more pronounced arboreal component. The low RLSI of A. afarensis does not imply a lack of arboreal activity given the evidence it climbed trees (Stern and Susman, 1983; Ruff et al., 2016; Green and Alemseged, 2012; DeSilva et al., 2018b). The glenoid:talus proportions of the DIK-1-1 juvenile are human-like and distinct from this ratio in modern apes or in other species of Australopithecus, despite the presence of a Gorilla-like scapula (Green and Alemseged, 2012) and medial cuneiform (DeSilva et al., 2018b) indicating increased arboreal competency among juveniles. Furthermore, although A. afarensis had a more modern human-like RLSI, it possessed relatively longer arms and shorter legs than modern humans (Holliday, 2012), with more chimpanzee-like humeral-femoral strength proportions (Ruff et al., 2016), suggesting that the body plan and positional repertoire of A. afarensis were unique and unlike any living taxon.
We acknowledge that one of the limitations of our study includes uncertainty surrounding the taxonomic affinity of partial skeletons such as KNM-ER 1500. However, our evolutionary interpretation would not be altered by accepting the alternative interpretation of KNM-ER 1500 as H. habilis. Our finding that Paranthropus had a high RLSI is consistent with recent evidence suggesting the presence of heavily built, somewhat ape-like, distal humeri in this genus (Lague et al., 2019). Additionally, for some specimens (e.g. KNM-ER 1503/1504, TM 1517), uncertainty remains about whether they represent a single partial skeleton, but recent work supports the single-individual hypothesis for TM 1517 (Cazenave et al., 2020). Finally, two skeletons used in this study are juveniles (MH1 and KNM-WT 15000), though they are near skeletal maturation. Despite their juvenile status, MH1 has more ape-like limb joint proportions, whereas KNM-WT 15000 is more modern human-like. Fortunately, the limb joint proportions of A. sediba are represented by the MH2 adult specimen. A future study could evaluate the relative size and ontogenetic scaling of limb joint proportions across hominoids to evaluate the morphometric and functional affinities of juvenile hominin specimens in greater detail (e.g., DIK-1-1).
Despite these minor caveats, the pattern of limb joint proportions in hominins is clear. Partial skeletons belonging to A. afarensis, H. erectus, and H. naledi are human-like, with a low RLSI, whereas all others are more ape-like, with a high RLSI. These data strongly suggest that A. afarensis was a committed terrestrial biped that evolved adaptations to limit the larger lower limb stresses and strains characteristic of bipedal locomotion, as also occurred in later Pleistocene Homo (but not H. floresiensis). Other species of Australopithecus, Paranthropus, and early members of the genus Homo appear to have been less committed terrestrial bipeds that retained adaptations to the arboreal milieu. Overall, our analysis provides resolution on a long-standing hypothesis that A. afarensis evolved its low RLSI independently of some later Pleistocene hominins.
Materials and methods
The comparative sample includes hylobatids (all four genera are represented, total N = 69; Hoolock: N = 11; Hylobates: N = 39; Nomascus: N = 5; Symphalangus: N = 14), Pongo spp. (total N = 50; Pongo abelii: N = 13; Pongo pygmaeus, N = 37), Gorilla spp. (total N = 131; Gorilla beringei: 55; Gorilla gorilla: N = 76), Pan spp. (total N = 124; Pan paniscus: N = 26, Pan troglodytes: N = 98), and H. sapiens (N = 67). We measured adult specimens from the Harvard Museum of Comparative Zoology, the American Museum of Natural History, and the Cleveland Museum of Natural History. Data for hominin partial skeletons (N = 16) were acquired from published literature and/or measured on original fossils using Mitutoyo calipers. In some cases, casts from the Dartmouth Paleoanthropology lab were used to confirm published measurements.
Seven measurements were taken at the shoulder and elbow joints to represent the upper body (Figure 5): scapular glenoid superoinferior (SI) height and maximum mediolateral (ML) width, humeral head SI height and anteroposterior (AP) width, humeral biepicondylar breadth, ulnar olecranon width, and radial head semimajor axis diameter. Seven measurements were taken at the hip, lumbosacral, and talocrural joints to represent the lower body (Figure 5): acetabulum SI height, SI femoral head diameter, femoral subtrochanteric ML width and AP breadth, sacral (S1) body maximum ML width and AP diameter at midline, and width of the talar trochlear apex taken at the midpoint. Mean scapular glenoid and humeral head joint size was calculated using a geometric mean of the SI and ML dimensions. Mean femoral subtrochanteric and sacral body size was calculated using a geometric mean of the AP and ML dimensions.
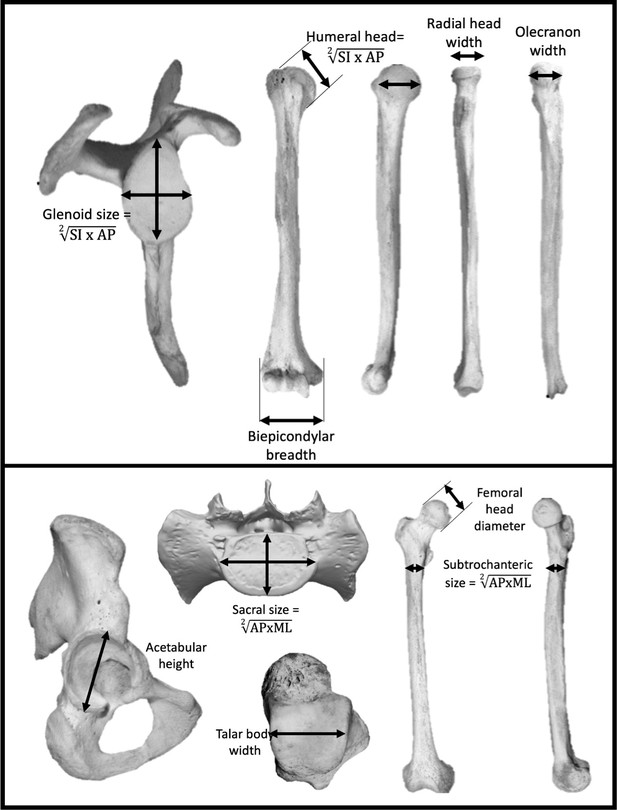
Linear measurements were taken on the upper limb (top) and lower limb (bottom).
Limb joint proportions were calculated using the relative limb size index, which is the logged ratio of geometric means calculated from forelimb and hindlimb measurements shown above (Green et al., 2007).
We quantified limb joint proportions per individual using the RLSI (Green et al., 2007). The RLSI is the logged ratio of geometric means calculated from forelimb and hindlimb measurements (Green et al., 2007). Geometric means are typically used as size proxies over arithmetic means because they accommodate measurements with different ranges, which is common for morphometric measurements, and therefore normalize the weight of individual measurements (Jungers et al., 1995). Logging the ratio is necessary because ratios of normally distributed data cannot be normally distributed and thus violate the assumptions of statistical tests (e.g., Green et al., 2007; Smith, 1999). The measurements used to calculate the limb joint proportions of hominin specimens varied depending on which measurements were preserved in the fossil (Supplementary file 1). Separate comparative analyses including different ratios were conducted for each hominin partial skeleton to maximize the fossil sample.
To visualize evolutionary scenarios, we conducted ancestral states using parsimony on a variety of phylogenetic hypotheses. These hypotheses included both formal cladistic analyses (Dembo et al., 2016; Strait et al., 2015; Mongle et al., 2019) and published hypotheses that have not been recovered in phylogenetic analyses (Asfaw et al., 1999; Wood and Schroer, 2017; Villmoare, 2018).
Data availability
All data generated during this study are included in the manuscript (Table 1). Raw data from extant specimens appears as an Excel file in Figure 2-source data 1.
References
-
Reassessment of the TM 1517 odonto-postcranial assemblage from Kromdraai B, South Africa, and the maturational pattern of Paranthropus robustusAmerican Journal of Physical Anthropology 172:714–722.https://doi.org/10.1002/ajpa.24082
-
New hominids from East Turkana, KenyaAmerican Journal of Physical Anthropology 45:369–435.https://doi.org/10.1002/ajpa.1330450304
-
The extremity bones of Paranthropus robustus from Kromdraai B, East formation member 3, Republic of South Africa: a reappraisalAnthropos 23:91–99.
-
BookPliocene Hominid Postcranial Fossils from the Middle Awash, EthiopiaBerkeley: University of California.
-
One small step: a review of Plio‐Pleistocene hominin foot evolutionAmerican Journal of Physical Anthropology 168:63–140.https://doi.org/10.1002/ajpa.23750
-
The position of Australopithecus sediba within fossil hominin hand use diversityNature Ecology & Evolution 4:911–918.https://doi.org/10.1038/s41559-020-1207-5
-
Plantigrady and foot adaptation in african apes: Implications for hominid originsAmerican Journal of Physical Anthropology 89:29–58.https://doi.org/10.1002/ajpa.1330890105
-
Climbing, brachiation, and terrestrial quadrupedalism: Historical precursors of hominid bipedalismAmerican Journal of Physical Anthropology 101:55–92.https://doi.org/10.1002/(SICI)1096-8644(199609)101:1<55::AID-AJPA5>3.0.CO;2-C
-
Limb joint surface areas and their ratios in malagasy lemurs and other mammalsAmerican Journal of Physical Anthropology 97:11–36.https://doi.org/10.1002/ajpa.1330970103
-
BookLimb proportions and positional behavior: Revisiting the theoretical and empirical underpinnings for locomotor reconstruction in Australopithecus africanusIn: Zipfel B, Richmond BG, Ward CV, editors. Hominid Postcranial Remains from Sterkfontein, South Africa, 1936-1995. Advances in Human Evolution Series. Oxford, United Kingdom: Oxford University Press. pp. 321–334.https://doi.org/10.1093/oso/9780197507667.001.0001
-
BookAssociated cranial and postcranial bones of Australopithecus boiseiIn: Grine FE, editors. Evolutionary History of the ‘“Robust”’ Australopithecines. Aldine de Gruyter. pp. 127–132.
-
Limb-size proportions in Australopithecus afarensis and Australopithecus africanusJournal of Human Evolution 52:187–200.https://doi.org/10.1016/j.jhevol.2006.09.001
-
Evolutionary reversals of limb proportions in early hominids? Evidence from KNM-ER 3735 (Homo habilisJournal of Human Evolution 53:383–405.https://doi.org/10.1016/j.jhevol.2007.06.001
-
In vivo baseline measurements of hip joint range of motion in suspensory and nonsuspensory anthropoidsAmerican Journal of Physical Anthropology 153:417–434.https://doi.org/10.1002/ajpa.22440
-
Was “Lucy” more human than her “child”? Observations on early hominid postcranial skeletonsJournal of Human Evolution 21:439–449.https://doi.org/10.1016/0047-2484(91)90094-C
-
Body size, body shape, and the circumscription of the genus HomoCurrent Anthropology 53:S330–S345.https://doi.org/10.1086/667360
-
Morphology of the Pliocene partial hominid skeleton (A.L. 288-1) from the Hadar formation, EthiopiaAmerican Journal of Physical Anthropology 57:403–451.https://doi.org/10.1002/ajpa.1330570403
-
Shape, relative size, and size-adjustments in morphometricsAmerican Journal of Physical Anthropology 38:137–161.https://doi.org/10.1002/ajpa.1330380608
-
Descriptions of the lower limb skeleton of Homo floresiensisJournal of Human Evolution 57:538–554.https://doi.org/10.1016/j.jhevol.2008.08.014
-
Man’s posture: Its evolution and disordersAm J Pub Health 13:580.https://doi.org/10.2105/ajph.13.7.580
-
Descriptions of the upper limb skeleton of Homo floresiensisJournal of Human Evolution 57:555–570.https://doi.org/10.1016/j.jhevol.2008.06.007
-
BookThe Fossil Hominids and an Introduction to Their Context. 1968-1974Oxford: Clarendon.
-
BookA partial skeleton of a gracile hominid from the upper Burgi member of the Koobi Fora formation, East Lake Turkana, KenyaIn: Giacobini G, editors. Hominidae: Proceedings of the 2nd International Congress of Human Paleontology Turin. Congresso Internazionale. pp. 167–174.
-
Evolution of human walkingScientific American 259:118–125.https://doi.org/10.1038/scientificamerican1188-118
-
Body size and proportions in early hominidsAmerican Journal of Physical Anthropology 87:407–431.https://doi.org/10.1002/ajpa.1330870404
-
Body proportions in Australopithecus afarensis and A. africanus and the origin of the genus HomoJournal of Human Evolution 35:1–22.https://doi.org/10.1006/jhev.1997.0197
-
Economy and endurance in human evolutionCurrent Biology 27:R613–R621.https://doi.org/10.1016/j.cub.2017.05.031
-
Conarticular congruence of the hominoid subtalar joint complex with implications for joint function in Plio-Pleistocene homininsAmerican Journal of Physical Anthropology 160:446–457.https://doi.org/10.1002/ajpa.22982
-
Hindlimb articular surface allometry in Hominoidea and Macaca, with comparisons to diaphyseal scalingJournal of Human Evolution 17:687–714.https://doi.org/10.1016/0047-2484(88)90025-5
-
Relative limb strength and locomotion in Homo habilisAmerican Journal of Physical Anthropology 138:90–100.https://doi.org/10.1002/ajpa.20907
-
Statistics of sexual size dimorphismJournal of Human Evolution 36:423–458.https://doi.org/10.1006/jhev.1998.0281
-
The locomotor anatomy of Australopithecus afarensisAmerican Journal of Physical Anthropology 60:279–317.https://doi.org/10.1002/ajpa.1330600302
-
Climbing to the top: A personal memoir of Australopithecus afarensisEvolutionary Anthropology: Issues, News, and Reviews 9:113–133.https://doi.org/10.1002/1520-6505(2000)9:3<113::AID-EVAN2>3.0.CO;2-W
-
BookAnalyzing hominin phylogeny: Cladistic approachIn: Henke W, Tattersall I, editors. Handbook of Paleoanthropology. Springer-Verlag. pp. 1989–2014.https://doi.org/10.1007/978-3-642-39979-4_58
-
Early Homo and the role of the genus in paleoanthropologyAmerican Journal of Physical Anthropology 165 Suppl 65:72–89.https://doi.org/10.1002/ajpa.23387
-
BookThe Nariokotome Homo erectus SkeletonBerlin, Heidelberg: Harvard University Press.
-
Morphology and evolution of the Homo naledi femora from LesediAmerican Journal of Physical Anthropology 170:5–23.
-
Interpreting the posture and locomotion of Australopithecus afarensis: Where do we stand?American Journal of Physical Anthropology 119:185–215.https://doi.org/10.1002/ajpa.10185
-
Evolution of the hominoid vertebral column: the long and the short of itEvolutionary Anthropology 24:15–32.https://doi.org/10.1002/evan.21437
-
The vertebrae, ribs, and sternum of Australopithecus sedibaPaleoAnthropology pp. 156–233.https://doi.org/10.4207/PA.2018.ART113
-
Paranthropus boisei: fifty years of evidence and analysisAmerican Journal of Physical Anthropology 134:106–132.https://doi.org/10.1002/ajpa.20732
-
BookParanthropus: Where do things stand?In: Marom A, Hovers E, editors. Human Paleontology and Prehistory. Vertebrate Paleobiology and Paleoanthropology. Springer. pp. 95–107.https://doi.org/10.1007/978-3-319-46646-0
Article and author information
Author details
Funding
Leakey Foundation
- Scott A Williams
Dartmouth College
- Jeremy M DeSilva
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Acknowledgements
The authors are grateful to the many individuals who made our data collection possible: Yohannes Haile-Selassie and Lyman Jellema (CMNH); Mark Omura (MCZ), Olivia Herschensohn, Kora Welsh, and Michèle Morgan (Harvard Peabody Museum of Archaeology and Ethnology); Neil Duncan, Eleanor Hoeger, Sara Ketelsen, Aja Marcato, Brian O’Toole, Marisa Surovy, and Eileen Westwig (AMNH); Emmanuel Gilissen and Wim Wendelen (Royal Museum for Central Africa); Lee Berger, Sifelani Jirah, and Bernhard Zipfel (Evolutionary Studies Institute, University of the Witwatersrand); Lazarus Kgasi, Stephany Potze, and Mirriam Tawane (Ditsong Museum of Natural History); Jared Assefa, Tomas Getachew, Getachew Senishaw, and Yonas Yilma (National Museum of Ethiopia, Authority for Research and Conservation of Cultural Heritage, and the Ethiopian Ministry of Culture and Tourism); Emma Mbua, Fredrick Manthi, and Job Kibii (National Museums of Kenya). We are additionally grateful to Matt Tocheri and Manuel Domínguez-Rodrigo for providing casts of hominin material included in this study.
Copyright
© 2021, Prabhat et al.
This article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use and redistribution provided that the original author and source are credited.
Metrics
-
- 3,549
- views
-
- 327
- downloads
-
- 16
- citations
Views, downloads and citations are aggregated across all versions of this paper published by eLife.
Citations by DOI
-
- 16
- citations for umbrella DOI https://doi.org/10.7554/eLife.65897






