CaV2.1 channel mutations causing familial hemiplegic migraine type 1 increase the susceptibility for cortical spreading depolarizations and seizures and worsen outcome after experimental traumatic brain injury
Figures
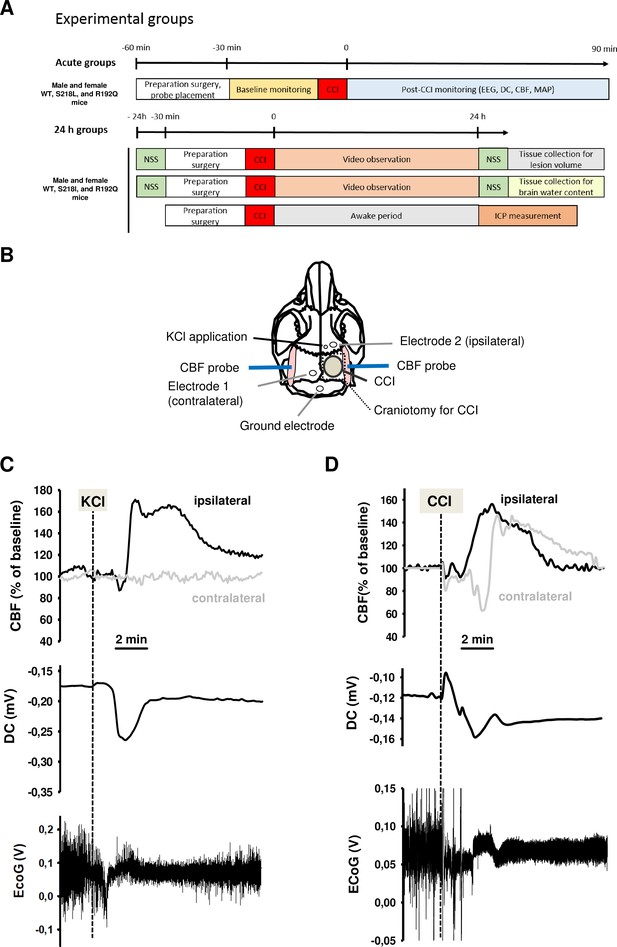
Design and methodology.
(A) Experimental plan. (B) Electrode placement. (C) Multiparametric recording of a cortical spreading depolarization (CSD) event elicited by application of 0.1 M potassium chloride (KCl): CSD is characterized by a negative direct current (DC) shift (middle panel, arrow), narrowing of electroencephalogram (EEG) amplitude (lower panel), and a consecutive cerebral hyperperfusion as indicated by an increase of cerebral blood flow (CBF) measured over the right hemisphere (ipsilateral to KCL application); CSD is restricted to the ipsilateral side, CBF on the contralateral hemisphere is not altered during CSD. (D) After controlled cortical impact (CCI), the DC shift is bidirectional, resulting in a pronounced change of EEG amplitude and is followed by a marked transient increase of cerebral perfusion first in the ipsilateral, then in the contralateral hemisphere.
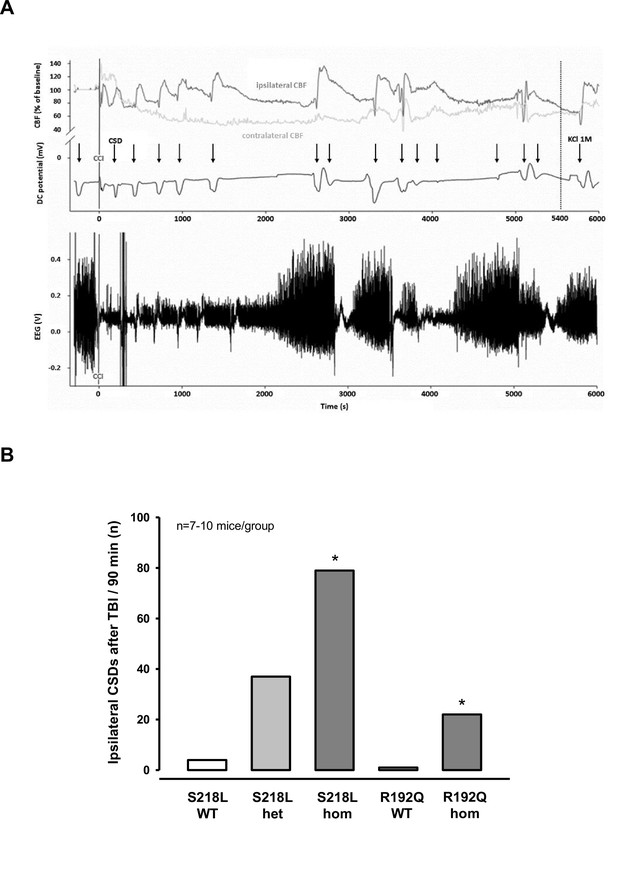
Cortical spreading depolarizations (CSDs) and electroencephalogram (EEG) in familial hemiplegic migraine type 1 (FHM1) mutant mice after trauma.
(A) Exemplary multiparametric recording of a (male) homozygous S218L mutant mouse showing multiple posttraumatic CSDs (black arrows). After the end of the observation period (90 min post controlled cortical impact (CCI), 5400 s), 2 µl 0.1 M KCl was applied to trigger a CSD event. The majority of posttraumatic CSDs were recorded ipsilateral to CCI as indicated by a hyperperfusion over the ipsilateral cortex (dark gray cerebral blood flow [CBF] trace). However, in some instances, sequential hyperperfusion of both hemispheres (corresponding to a bihemispheric CSD) or an isolated contralateral hyperperfusion (corresponding to CSD originating in the contralateral, i.e., nontraumatized side) was observed after direct current (DC) and EEG changes characteristic for a CSD event. (B) After CCI, all mutant mice show a highly increased propensity for CSDs: S218L mutants showed a much higher frequency of CSD events during the 90 min observation time as compared to wild-type (WT) littermates (p < 0.001 S218L hom. vs. S218L WT, analysis of variance [ANOVA] on ranks). Also in the R192Q strain, homozygous mice experienced more CSDs than WT mice (p < 0.01 R192Q hom. vs. R192Q WT, ANOVA on ranks). S218L WT: n = 8; S218L het.: n = 10; S218L hom.: n = 7; R192Q WT: n = 10; R192Q hom.: n = 10.
-
Figure 2—source data 1
Figure 2B- Ipsilateral CSDs after TBI.
- https://cdn.elifesciences.org/articles/74923/elife-74923-fig2-data1-v3.xlsx
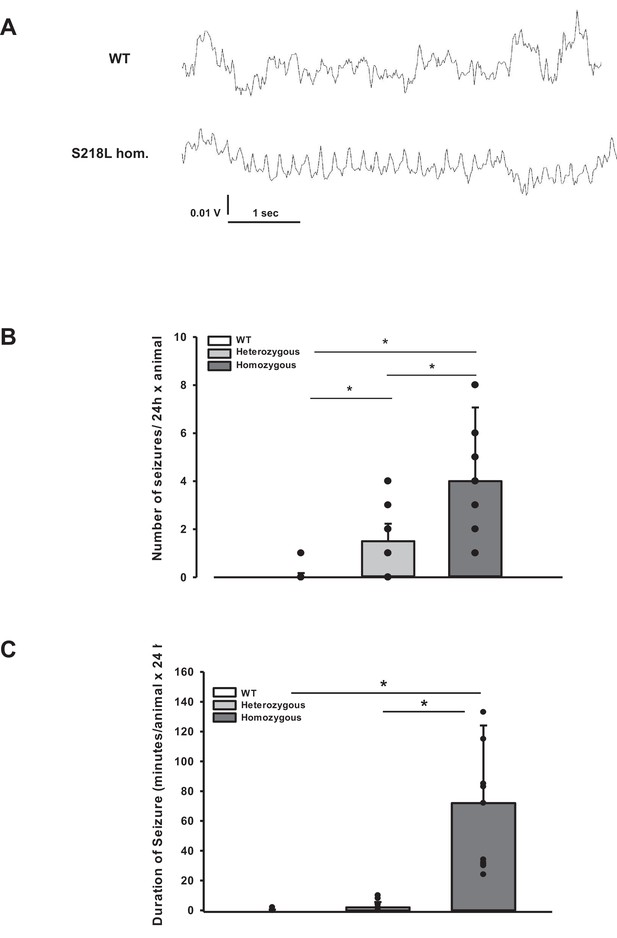
Posttraumatic seizures in familial hemiplegic migraine type 1 (FHM1) mutant mice after controlled cortical impact (CCI).
(A) Exemplary sections of the electroencephalogram (EEG) trace of a homozygous S218L mutant mouse: While EEG showed a normal pattern during baseline monitoring (upper curve), synchronized EEG activity with a frequency of 4–8 Hz as shown in the lower trace was recorded in S218L mutants only; during these phases mice intermittently showed tonic tail contraction and/or opisthotonus suggestive for seizure activity. (B) While seizures occurred very rarely in wild-type (WT) mice (one event in total group), S218L mutants experienced a high number of seizures. The total number of epileptic seizures per 24 hr was significantly higher in homozygous S218L mutant than in heterozygous S218L mutant and WT mice (n = 16–18 each; median ± 95% confidence interval, *p < 0.001, analysis of variance [ANOVA] on ranks). (C) Average duration of seizure activity in minutes per animal within the first 24 hr after CCI. Homozygous S218L mutants on average experienced 72 min of seizure activity in 24 hr while the few seizures occurring in heterozygous mutant and WT mice lasted significantly shorter (n = 11 each; median ± 95% confidence interval, *p ≤ 0.001, ANOVA on ranks).
-
Figure 3—source data 1
Figure 3B, C Number and duraration of seizures after TBI.
- https://cdn.elifesciences.org/articles/74923/elife-74923-fig3-data1-v3.xlsx
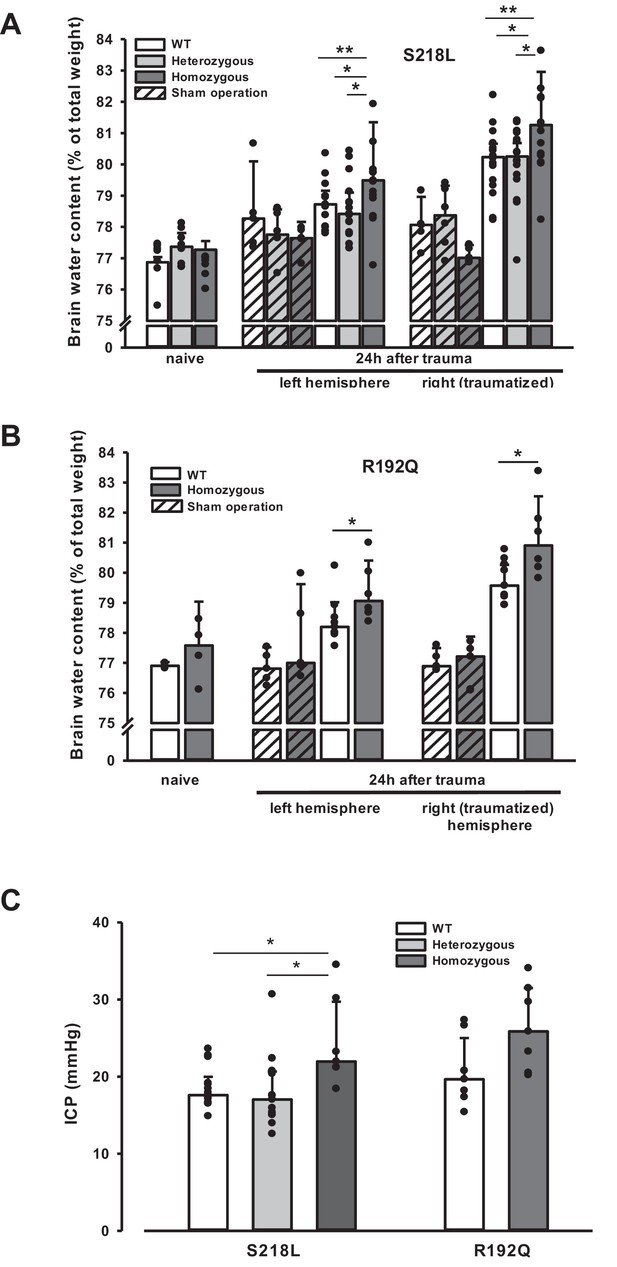
Brain edema formation in familial hemiplegic migraine type 1 (FHM1) mutant mice 24 hr after controlled cortical impact (CCI).
(A) Pretrauma brain water content (naive) is approximately 77.5% and comparable in all genotypes; sham operation (striped bars) does not induce significant changes of brain water content. Twenty-four hours after CCI, brain water content in the traumatized (right) hemisphere is significantly increased compared to the respective controls; in homozygous S218L mutant mice (dark gray bars) brain edema formation is significantly more pronounced than in wild-type (WT; white bars) and S218L heterozygous mutant mice (light gray bars). Heterozygous animals, in contrast, showed no difference to WT mice. In the contralateral/nontraumatized hemisphere only homozygous S218L mutant mice had a significant increase in brain water content. As in the traumatized hemisphere, brain water content was significantly higher in homozygous S218L mutants than in WT and heterozygous S218L mutant mice (sham/naive: n = 5–8/group; S218L hom./het.: n = 15–18 each; WT: n = 16; median ± 95% confidence interval, *p < 0.05, **p < 0.005 vs. naive brain water content, analysis of variance [ANOVA] on ranks). (B) In the R192Q strain, brain water content was within normal limits in naive and sham-operated animals; 24 hr after CCI, however, it was significantly increased in homozygous mutant mice (dark gray bars) in both hemispheres compared to WT (white bars) (Sham/naive: n = 4–5/group; R192Q hom.: n = 6; WT: n = 9; median ± 95% confidence interval, *p < 0.05, rank sum test). (C) Intracranial pressure (ICP) 24 hr after trauma was significantly elevated in S218L homozygous mice (left panel, dark gray bar) compared to WT (white bar) and heterozygous mutant mice (light gray bar); in the R192Q strain, ICP tended to be higher in mutant animals (right panel, dark gray bar) than in their WT littermates (white bar; S218L het.: n = 15; S218L hom.: n = 7; S218L WT: n = 15; R192Q hom.: n = 7; R192Q WT: n = 7; median ± 95% confidence interval, *p < 0.005, ANOVA on ranks).
-
Figure 4—source data 1
Figure 4 Brain edema formation after TBI.
- https://cdn.elifesciences.org/articles/74923/elife-74923-fig4-data1-v3.xlsx
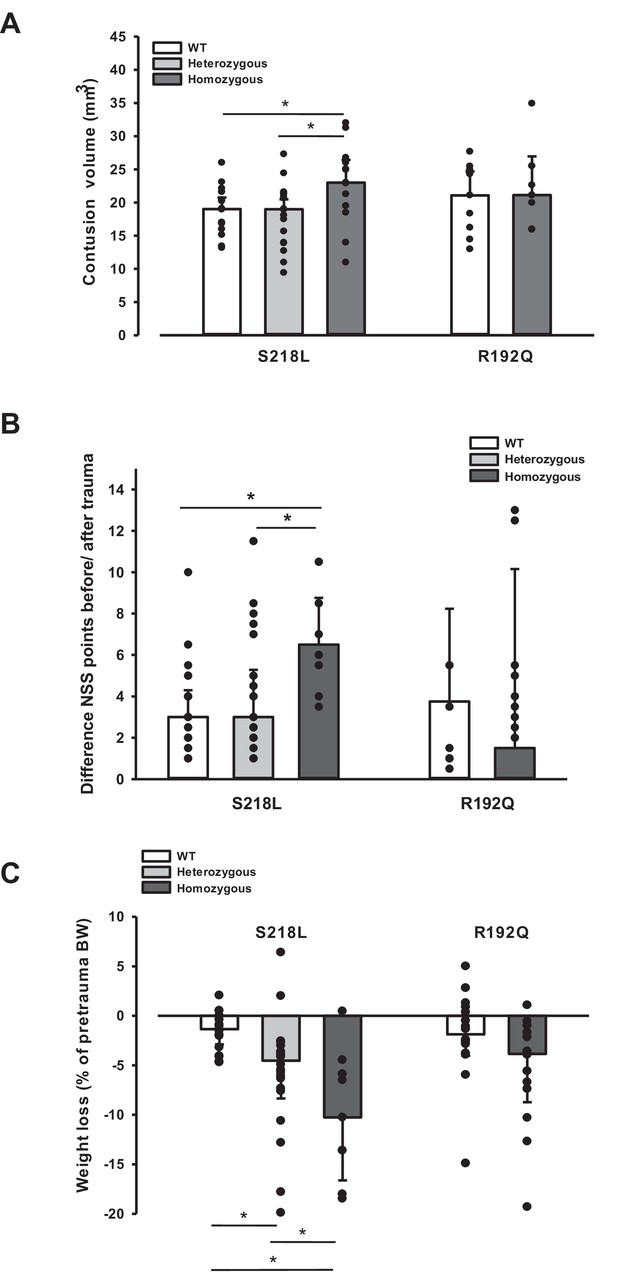
Brain damage and functional outcome in familial hemiplegic migraine type 1 (FHM1) mutant mice 24 hr after controlled cortical impact (CCI).
(A) Volume of structural posttraumatic brain damage, that is contusion volume, was significantly higher in homozygous S218L mutant mice compared to heterozygous S218L mutant and wild-type (WT) animals; in the R192Q strain, lesion volume was only increased by trend (S218L hom./het.: n = 13–21 each; WT: n = 16; R192Q hom.: n = 9, WT: n = 10; median ± 95% confidence interval, *p < 0.5, analysis of variance [ANOVA] on ranks). (B) Performance in a multivariate neurological test (maximum deficit 20 points), expressed by differences of points obtained before and 24 hr after traumatic brain injury (TBI). Higher scores indicate higher deficits, that is healthy animals usually score 0–1.5 points. Homozygous S218L mutant mouse’s scores deteriorated significantly more than in the heterozygous S218L mutant or WT group (left panel). In the R192Q mutants, performance was comparable to WT mice (S218L hom./het.: n = 10–23 each; WT: n = 23; R192Q hom.: n = 9; WT: n = 10; median ± 95% confidence interval, *p < 0.005, ANOVA on ranks, Dunn’s post hoc test). (C) Weight loss 24 hr after CCI: heterozygous and homozygous S218L mutant mice lost significantly and gene dose dependently more weight, R192Q animals’ weight did not differ from their WT littermates (S218L hom./het.: n = 9–22; WT: n = 21; R192Q hom.: n = 14; WT: n = 18; median ± 95% confidence interval, *p < 0.05, ANOVA on ranks, Dunn’s post hoc test).
-
Figure 5—source data 1
Figure 5 Brain damage and functional outcome in FHM1 mutant mice after TBI.
- https://cdn.elifesciences.org/articles/74923/elife-74923-fig5-data1-v3.xlsx





