Listeria monocytogenes requires cellular respiration for NAD+ regeneration and pathogenesis
Figures
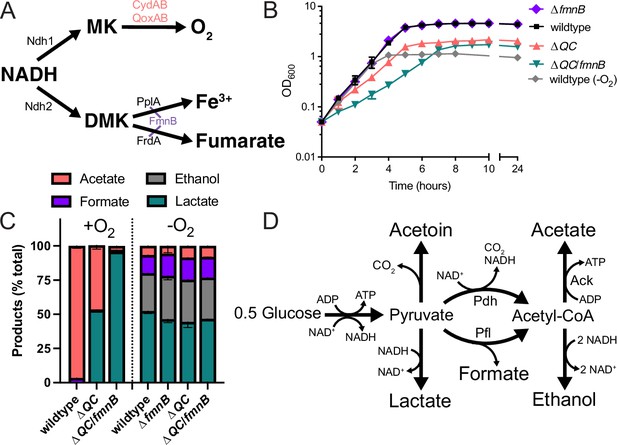
Respiration impacts L. monocytogenes growth and fermentative output.
(A) Proposed respiratory electron transport chains in L. monocytogenes. Different NADH dehydrogenases likely transfer electrons to distinct but presently unidentified quinones (Qa and Qb). FmnB catalyzes assembly of essential components of the electron transport chain, PplA and FrdA, that can transfer electrons to ferric iron and fumarate, respectively. Other proteins involved in the terminal electron transfer steps are noted. (B) Optical density of L. monocytogenes strains aerobically grown in nutrient-rich media, with the anaerobically grown wildtype strain provided for context. The means and standard deviations from three independent experiments are shown. (C) Fermentation products of L. monocytogenes strains grown to stationary phase in nutrient-rich media under aerobic and anaerobic conditions. Error bars show standard deviations. Results from three independent experiments are shown. (D) Proposed pathways for L. monocytogenes sugar metabolism. The predicted number of NADH generated (+) or consumed (−) in each step is indicated. PplA, peptide pheromone-encoding lipoprotein A; FrdA, fumarate reductase; ΔQC, ΔqoxA/ΔcydAB; ΔQC/fmnB, ΔqoxA/ΔcydAB/fmnB::tn; GLC, glucose; Ack, acetate kinase; Pdh, pyruvate dehydrogenase; Pfl, pyruvate formate-lyase; DMK, demethylmenaquinone.
-
Figure 1—source data 1
Source data for Figure 1B.
- https://cdn.elifesciences.org/articles/75424/elife-75424-fig1-data1-v2.xlsx
-
Figure 1—source data 2
Source data for Figure 1C.
- https://cdn.elifesciences.org/articles/75424/elife-75424-fig1-data2-v2.xlsx
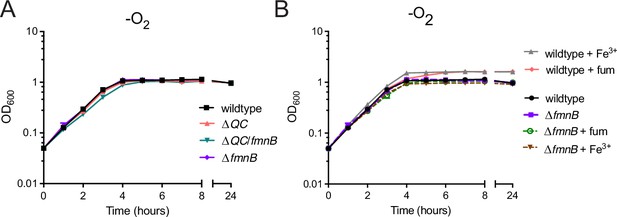
Use of respiratory electron acceptors enhances Listeria monocytogenes growth in nutrient-rich media.
(A) Optical density of L. monocytogenes strains grown anaerobically in nutrient-rich media. The data represent the means and standard deviations from three independent experiments. (B) Optical density of anaerobically grown strains in nutrient-rich media supplemented with the alternative electron acceptors ferric iron (Fe3+) or fumarate (fum), as indicated. The means and standard deviations from three independent experiments are shown.
-
Figure 1—figure supplement 1—source data 1
Source data for Figure 1—figure supplement 1A.
- https://cdn.elifesciences.org/articles/75424/elife-75424-fig1-figsupp1-data1-v2.xlsx
-
Figure 1—figure supplement 1—source data 2
Source data for Figure 1—figure supplement 1B.
- https://cdn.elifesciences.org/articles/75424/elife-75424-fig1-figsupp1-data2-v2.xlsx
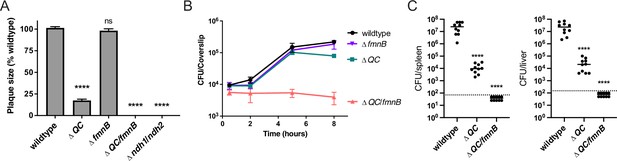
Respiration is required for L. monocytogenes virulence.
(A) Plaque formation by cell-to-cell spread of L. monocytogenes strains in monolayers of mouse L2 fibroblast cells. The mean plaque size of each strain is shown as a percentage relative to the wildtype plaque size. Error bars represent standard deviations of the mean plaque size from two independent experiments. Statistical analysis was performed using one-way ANOVA and Dunnett’s post-test comparing wildtype to all the other strains. ****, p<0.0001; ns, no significant difference (p>0.05). (B) Intracellular growth of L. monocytogenes strains in murine bone marrow-derived macrophages (BMMs). At 1-hour post-infection, infected BMMs were treated with 50 μg/mL of gentamicin to kill extracellular bacteria. Colony-forming units (CFU) were enumerated at the indicated times. Results are representative of two independent experiments. (C) Bacterial burdens in murine spleens and livers 48 hours post-intravenous infection with indicated L. monocytogenes strains. The median values of the CFUs are denoted by black bars. The dashed lines represent the limit of detection. Data were combined from two independent experiments, n = 10 mice per strain. Statistical significance was evaluated using one-way ANOVA and Dunnett’s post-test using wildtype as the control. ****, p<0.0001. ΔQC, ΔqoxA/ΔcydAB; ΔQC/fmnB, ΔqoxA/ΔcydAB/fmnB::tn; Δndh1/ndh2, Δndh1/ndh2::tn.
-
Figure 2—source data 1
Source data for Figure 2A.
- https://cdn.elifesciences.org/articles/75424/elife-75424-fig2-data1-v2.xlsx
-
Figure 2—source data 2
Source data for Figure 2B.
- https://cdn.elifesciences.org/articles/75424/elife-75424-fig2-data2-v2.xlsx
-
Figure 2—source data 3
Source data for Figure 2C.
- https://cdn.elifesciences.org/articles/75424/elife-75424-fig2-data3-v2.xlsx
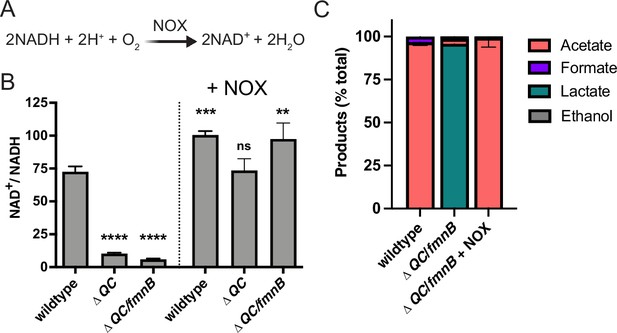
Water-forming NADH oxidase (NOX) restores redox homeostasis in respiration-deficient L. monocytogenes strains.
(A) Reaction catalyzed by the Lactococcus lactis water-forming NOX, which is the same as aerobic respiration without the generation of a proton motive force. (B) NAD+/NADH ratios of parent and NOX-complemented L. monocytogenes strains grown aerobically in nutrient-rich media to mid-logarithmic phase. Results from three independent experiments are presented as means and standard deviations. Statistical significance was calculated using one-way ANOVA and Dunnett’s post-test using the wildtype parent strain as the control. ****, p<0.0001; ***, p<0.001; **, p<0.01; ns, not statistically significant (p>0.05). (C) Fermentation products of L. monocytogenes strains grown in nutrient-rich media under aerobic conditions. Error bars show standard deviations. Results from three independent experiments are shown. ΔQC, ΔqoxA/ΔcydAB; ΔQC/fmnB, ΔqoxA/ΔcydAB/fmnB::tn; + NOX, strains complemented with L. lactis nox.
-
Figure 3—source data 1
Source data for Figure 3B.
- https://cdn.elifesciences.org/articles/75424/elife-75424-fig3-data1-v2.xlsx
-
Figure 3—source data 2
Source data for Figure 3C.
- https://cdn.elifesciences.org/articles/75424/elife-75424-fig3-data2-v2.xlsx
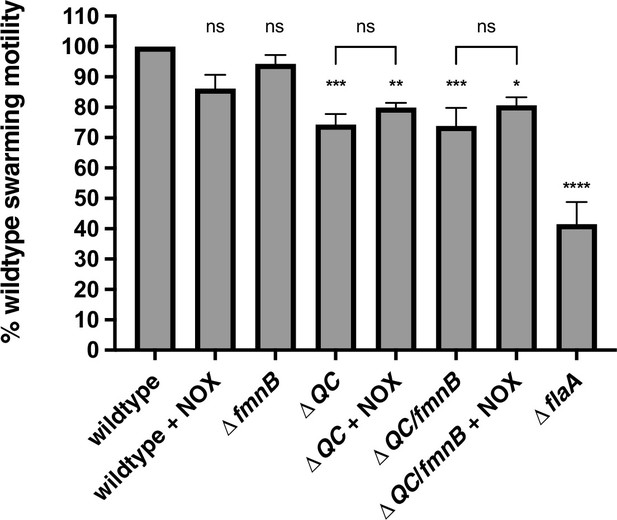
NOX expression in respiration-deficient mutants fails to rescue swarming motility.
The swarming motility of parent and NOX-complemented Listeria monocytogenes strains is shown as a percentage relative to the wildtype swarming diameter following 48 hours incubation at 30°C. Error bars represent standard deviations of the mean swarming diameters from three independent experiments. Statistical significance between the wildtype and mutant strains was calculated using one-way ANOVA and unpaired two-tailed t test was utilized to determine significance between parent and NOX-complemented strains. ****, p<0.0001; ***, p<0.001; **, p<0.01; *, p<0.05; ns, no significant difference (p>0.05).
-
Figure 3—figure supplement 1—source data 1
Source data for Figure 3—figure supplement 1.
- https://cdn.elifesciences.org/articles/75424/elife-75424-fig3-figsupp1-data1-v2.xlsx
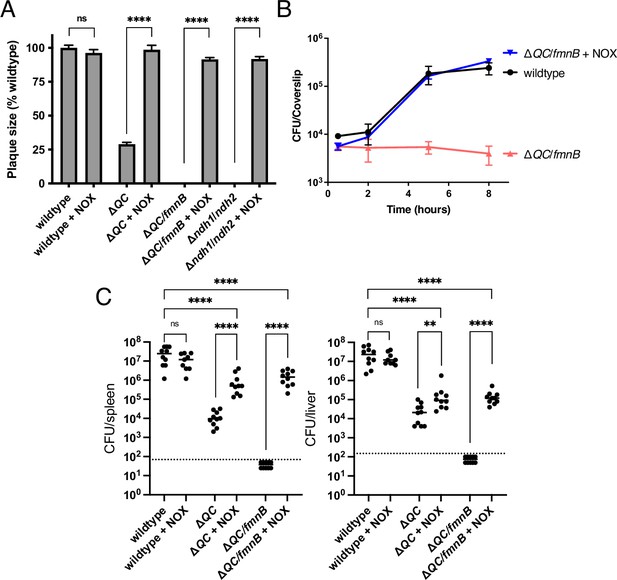
NOX expression restores virulence to respiration-deficient L. monocytogenes strains.
(A) Plaque formation by cell-to-cell spread of L. monocytogenes strains in monolayers of mouse L2 fibroblast cells. The mean plaque size of each strain is shown as a percentage relative to the wildtype plaque size. Error bars represent standard deviations of the mean plaque size from two independent experiments. Statistical analysis was performed using the unpaired two-tailed t test. ****, p<0.0001; ns, no significant difference (p>0.05). (B) Intracellular growth of L. monocytogenes strains in murine bone marrow-derived macrophages (BMMs). At 1-hour post-infection, infected BMMs were treated with 50 μg/mL of gentamicin to kill extracellular bacteria. Colony-forming units (CFU) were enumerated at the indicated times. Results are representative of three independent experiments. (C) Bacterial burdens in murine spleens and livers 48 hours post-intravenous infection with indicated L. monocytogenes strains. The median values of the CFUs are denoted by black bars. The dashed lines represent the limit of detection. Data were combined from two independent experiments, n = 10 mice per strain, but for the wildtype +NOX strain (n = 9 mice). Statistical significance was evaluated using one-way ANOVA and Dunnett’s post-test using the wildtype control strain to compare with the NOX-complemented strains. Significance between the parental and the NOX-complemented strains was determined using the unpaired two-tailed t test. ****, p<0.0001; **, p<0.01; ns, no significant difference (p>0.05). ΔQC, ΔqoxA/ΔcydAB; ΔQC/fmnB, ΔqoxA/ΔcydAB/fmnB::tn; Δndh1/ndh2, Δndh1/ndh2::tn; + NOX, strains complemented with Lactococcus lactis nox.
-
Figure 4—source data 1
Source data for Figure 4A.
- https://cdn.elifesciences.org/articles/75424/elife-75424-fig4-data1-v2.xlsx
-
Figure 4—source data 2
Source data for Figure 4B.
- https://cdn.elifesciences.org/articles/75424/elife-75424-fig4-data2-v2.xlsx
-
Figure 4—source data 3
Source data for Figure 4C.
- https://cdn.elifesciences.org/articles/75424/elife-75424-fig4-data3-v2.xlsx
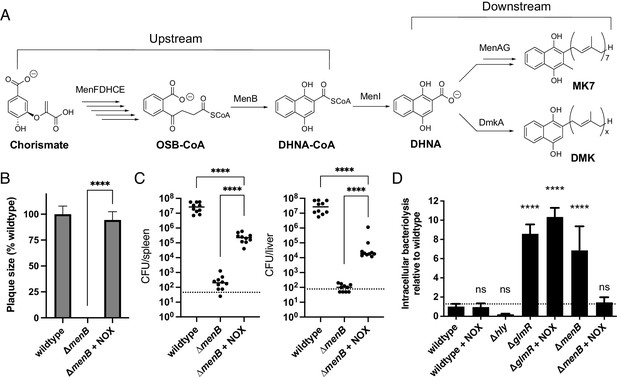
Impaired redox homeostasis accounts for elevated bacteriolysis of a respiration-deficient L. monocytogenes strain in the cytosol of infected cells.
(A) Proposed L. monocytogenes quinone biosynthesis pathway. Arrows indicate the number of enzymes that catalyze each reaction. An unidentified demethylmenaquinone (DMK) is proposed to be required for the flavin-based electron transfer pathway and MK7 required for aerobic respiration. Loss of the upstream portion of the pathway is anticipated to impact both electron transport chains. (B) Plaque formation by cell-to-cell spread of L. monocytogenes strains in monolayers of mouse L2 fibroblast cells. The mean plaque size of each strain is shown as a percentage relative to the wildtype plaque size. Error bars represent standard deviations of the mean plaque size from two independent experiments. Statistical analysis was performed using the unpaired two-tailed t test. ****, p<0.0001. (C) Bacterial burdens in murine spleens and livers 48 hours post-intravenous infection with indicated L. monocytogenes strains. The median values of the CFUs are denoted by black bars. The dashed lines represent the limit of detection. Data were combined from two independent experiments, n = 10 mice per strain. Statistical significance was evaluated using one-way ANOVA and Dunnett’s post-test using the wildtype strain as the control to compare with the NOX-complemented strain. Significance between the parental and the NOX-complemented strain was determined using the unpaired two-tailed t test. ****, p<0.0001. (D) Bacteriolysis of L. monocytogenes strains in bone marrow-derived macrophages. The data are normalized to wildtype bacteriolysis levels and presented as means and standard deviations from three independent experiments. Statistical significance was calculated using one-way ANOVA and Dunnett’s post-test using the wildtype parent strain as the control. ****, p<0.0001; ns, no significant difference (p>0.05).
-
Figure 5—source data 1
Source data for Figure 5B.
- https://cdn.elifesciences.org/articles/75424/elife-75424-fig5-data1-v2.xlsx
-
Figure 5—source data 2
Source data for Figure 5C.
- https://cdn.elifesciences.org/articles/75424/elife-75424-fig5-data2-v2.xlsx
-
Figure 5—source data 3
Source data for Figure 5D.
- https://cdn.elifesciences.org/articles/75424/elife-75424-fig5-data3-v2.xlsx
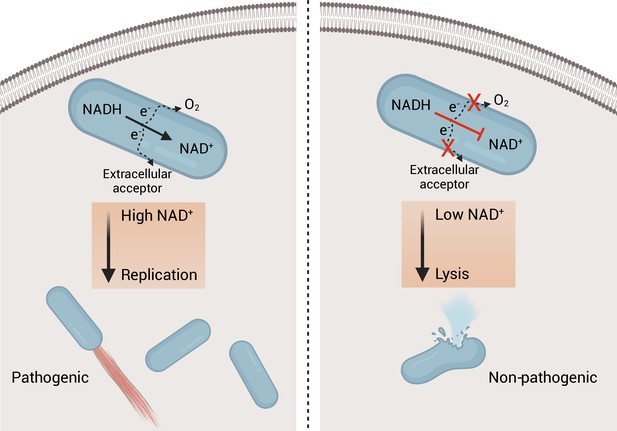
Model of the role of respiration in L. monocytogenes pathogenesis.
On the left, an intracellular bacterium with the ability to oxidize NADH and transfer electrons through the aerobic and extracellular electron transfer electron transport chains can regenerate and maintain high NAD+ levels allowing the bacterium to grow and be virulent. On the right, an intracellular bacterium unable to regenerate NAD+, by lacking the electron transport chains, is avirulent because it lyses in the cytosol of infected cells.
Tables
Bacterial strains used in this study.
| Strains | Strain number | Reference |
|---|---|---|
| Listeria monocytogenes (wildtype) | 10403S | Bécavin et al., 2014 |
| ΔcydAB/ΔqoxA | DP-L6624 | Chen et al., 2017 |
| ΔcydAB/ΔqoxA/fmnB::tn | DP-L7190 | This study |
| ΔfmnB | DP-L7195 | This study |
| Wildtype + pPL2 NOX | DP-L7188 | This study |
| ΔcydAB/ΔqoxA + pPL2 NOX | DP-L7189 | This study |
| ΔcydAB/ΔqoxA/fmnB::tn + pPL2 NOX | DP-L7191 | This study |
| ΔflaA | DP-L5986 | Nguyen et al., 2020 |
| Δndh1/ndh2::tn | DP-L6626 | This study |
| Δndh1/ndh2::tn + pPL2 NOX | DP-L7253 | This study |
| Wildtype + pBHE573 | JDS18 | Sauer et al., 2010 |
| Wildtype + pPL2 NOX + pBHE573 | JDS2328 | This study |
| ΔmenB + pBHE573 | JDS1191 | Chen et al., 2017 |
| ΔmenB + pPL2 NOX + pBHE573 | JDS2333 | This study |
| Δhly + pBHE573 | JDS19 | Sauer et al., 2010 |
| ΔglmR + pBHE573 | JDS21 | Sauer et al., 2010 |
| ΔglmR + pPL2 NOX + pBHE573 | JDS2329 | This study |
| Escherichia coli | SM10 | |
| pPL2-NOX | DP-E7206 | This study |
| pBHE573 | JDS17 | Sauer et al., 2010 |





