Neuronal temperature perception induces specific defenses that enable C. elegans to cope with the enhanced reactivity of hydrogen peroxide at high temperature
Figures
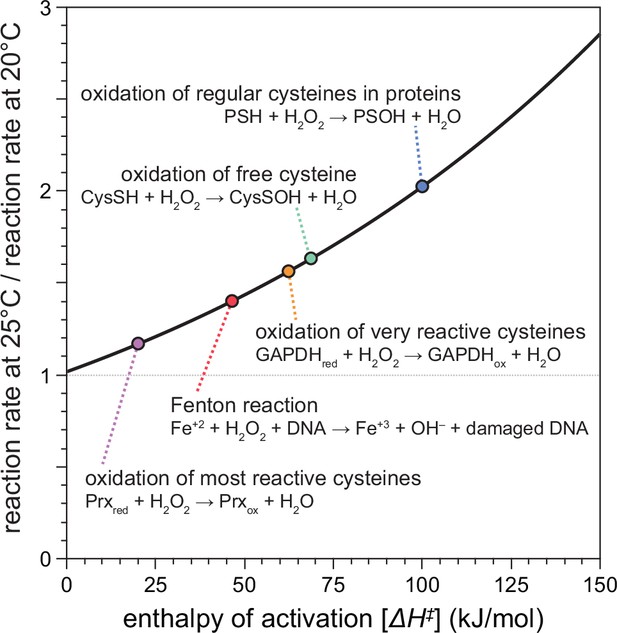
Temperature-dependent enhancement of H2O2 reactivity toward biologically important molecules.
Using chemical kinetics, we modeled how much the rates of chemical reactions of H2O2 change between 20 and 25°C. This plot illustrates how the enthalpy of activation of a specific chemical reaction influences its rate at 25°C compared to 20°C. The dotted arrows point to the relative reaction rate values for specific reactions of H2O2 with biologically important molecules.
-
Figure 1—source data 1
Kinetic modeling data.
- https://cdn.elifesciences.org/articles/78941/elife-78941-fig1-data1-v2.xlsx
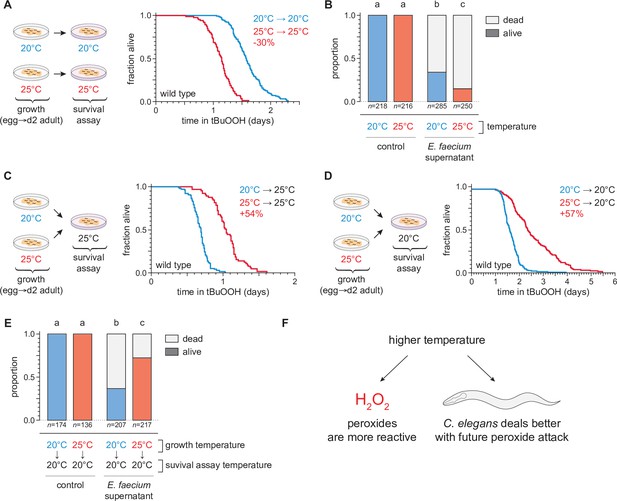
Temperature regulates the peroxide resistance of C. elegans.
(A) Peroxide resistance of wild-type C. elegans grown and assayed at 20 or 25°C. The fraction of nematodes remaining alive in the presence of 6 mM tert-butyl hydroperoxide (tBuOOH) is plotted against time. (B) Survival of wild-type C. elegans 16 hr after exposure to E. faecium E007 liquid-culture supernatant. Nematodes were grown and assayed at 20 or 25°C. Groups labeled with different letters exhibited significant survival differences (p < 0.001, ordinal logistic regression) otherwise (p > 0.05). (C) Peroxide resistance at 25°C of wild-type C. elegans grown at 20 or 25°C. (D) Peroxide resistance at 20°C of wild-type C. elegans grown at 20 or 25°C. (E) Survival of wild-type C. elegans 16 hr after exposure to E. faecium E007 liquid-culture supernatant. Nematodes were grown at 20 or 25°C and assayed at 20°C. Groups labeled with different letters exhibited significant survival differences (p < 0.001, ordinal logistic regression) otherwise (p > 0.05). (F) Peroxides killed C. elegans more quickly at 25°C than at 20°C, but nematodes grown at 25°C could survive a subsequent peroxide exposure better than those grown at 20°C. Statistical analyses for panels (A, C, and D) are in Supplementary file 1.
-
Figure 2—source data 1
Survival data for panels A, C, D.
- https://cdn.elifesciences.org/articles/78941/elife-78941-fig2-data1-v2.xlsx
-
Figure 2—source data 2
Survival data for panels B, E.
- https://cdn.elifesciences.org/articles/78941/elife-78941-fig2-data2-v2.xlsx
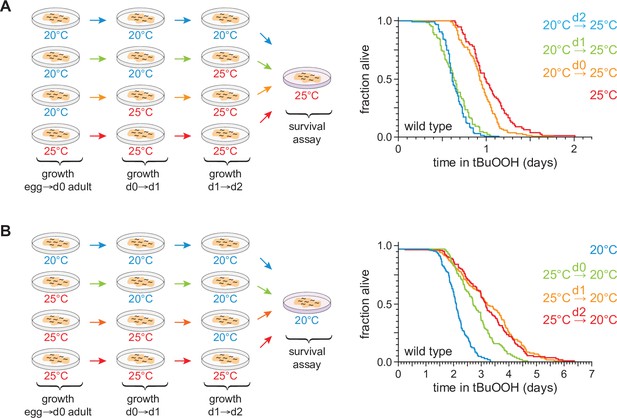
C.elegans can induce lasting peroxide defenses in response to high temperature.
(A) Peroxide resistance at 25°C of wild-type C. elegans shifted from 20 to 25°C at the specified time before the assay. (B) Peroxide resistance at 20°C of wild-type C. elegans shifted from 25 to 20°C at the specified time before the assay. Statistical analyses for panels (A, B) are in Supplementary file 1.
-
Figure 2—figure supplement 1—source data 1
Survival data for panels A, B.
- https://cdn.elifesciences.org/articles/78941/elife-78941-fig2-figsupp1-data1-v2.xlsx
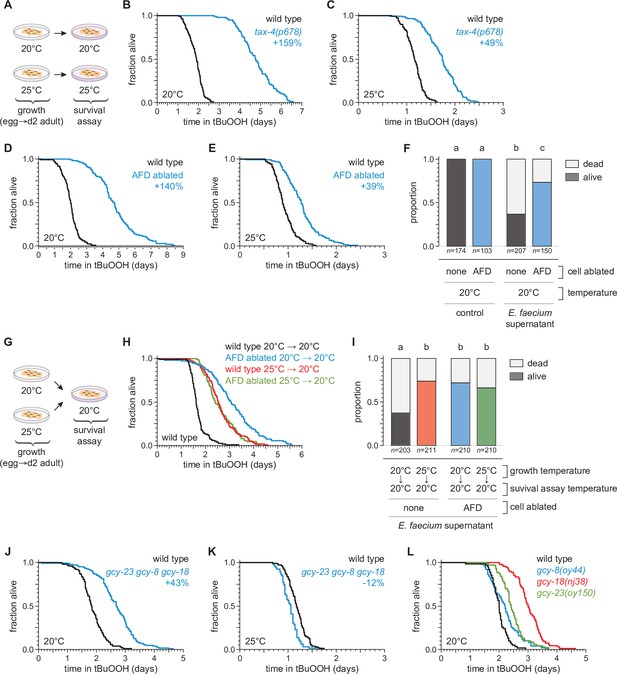
The AFD sensory neurons are required for the temperature dependence of C.elegans peroxide resistance.
(A) Diagram summarizing the experimental strategy for panels (B–F and J–L). The tax-4(p678) mutation increased peroxide resistance by a greater factor at 20°C (B) than at 25°C (C). Genetic ablation of AFD increased peroxide resistance by a greater factor at 20°C (D) than at 25°C (E). (F) Genetic ablation of AFD increased the proportion of nematodes that survived 16 hr after exposure to E. faecium E007 liquid-culture supernatant. Nematodes were grown and assayed at 20°C. Groups labeled with different letters exhibited significant differences (p < 0.001, ordinal logistic regression) otherwise (p > 0.05). (G) Diagram summarizing the experimental strategy for panels (H, I). (H) AFD-ablated nematodes grown at 25°C did not exhibit a further increase in peroxide resistance at 20°C, unlike wild-type (unablated) nematodes. (I) Growth at 25°C did not further increase the proportion of AFD-ablated nematodes that survived after 16-hr exposure to E. faecium E007 liquid-culture supernatant at 20°C, unlike in wild-type (unablated) nematodes. Groups labeled with different letters exhibited significant differences (p < 0.001, ordinal logistic regression) otherwise (p > 0.05). (J–K) gcy-23(oy150) gcy-8(oy44) gcy-18(nj38) triple null mutants show increased peroxide resistance at 20°C (J) and decreased peroxide resistance at 25°C (J). (L) Peroxide resistance of gcy-8(oy44), gcy-18(nj38), and gcy-23(oy150) single mutants and wild-type nematodes at 20°C. Statistical analyses for panels (B–E, H, J–L) are in Supplementary file 2.
-
Figure 3—source data 1
Survival data for panels B–E, H, J–L.
- https://cdn.elifesciences.org/articles/78941/elife-78941-fig3-data1-v2.xlsx
-
Figure 3—source data 2
Survival data for panels F, I.
- https://cdn.elifesciences.org/articles/78941/elife-78941-fig3-data2-v2.xlsx
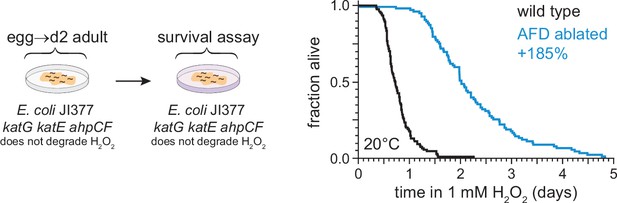
The AFD sensory neurons regulate C. elegans H2O2 resistance.
Hydrogen peroxide resistance of wild-type and AFD-ablated C. elegans nematodes at 20°C. The nematodes were grown and assayed on lawns of E. coli JI377, a katG katE ahpCF triple null mutant strain unable to degrade H2O2 in the environment (Seaver and Imlay, 2001). Statistical analysis for panel (A) is in Supplementary file 2.
-
Figure 3—figure supplement 1—source data 1
Survival data.
- https://cdn.elifesciences.org/articles/78941/elife-78941-fig3-figsupp1-data1-v2.xlsx
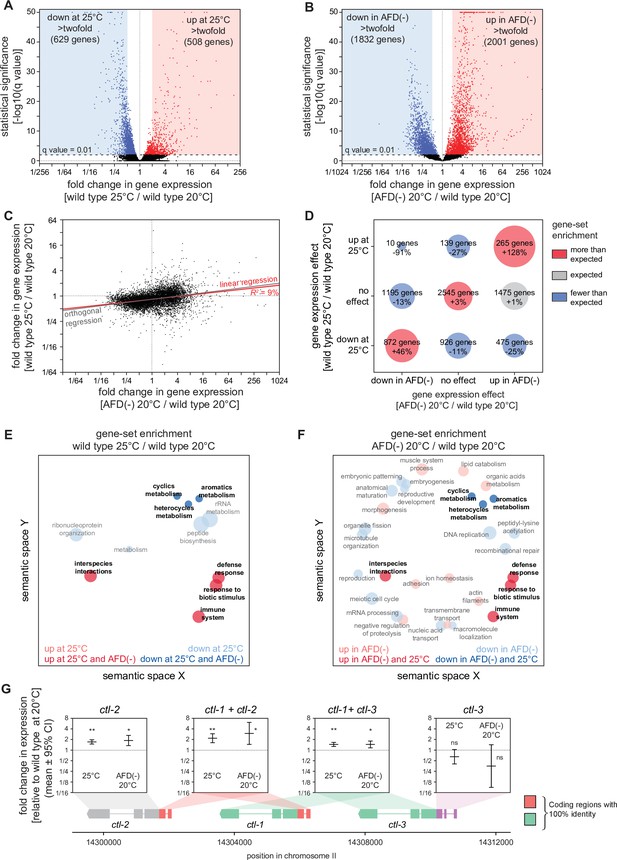
Hydrogen peroxide defenses are induced by high cultivation temperature and AFD ablation.
Volcano plots showing the level and statistical significance of changes in gene expression induced (A) in wild-type nematodes by growth at 25°C relative to growth at 20°C and (B) by AFD ablation in nematodes grown at 20°C relative to wild-type (unablated) nematodes grown at 20°C. Genes up- and downregulated significantly (q value <0.01) are shown in red and blue, respectively. (C) Growth at 25°C and AFD ablation at 20°C induced correlated changes in gene expression. Linear regression fit is shown as a red line flanked by a red area marking the 95% confidence interval of the fit. The orthogonal regression fit (gray line) makes no assumptions about the dependence or independence of the variables. (D) Coregulation of genes up- and downregulated significantly (q value <0.01) by growth at 25°C and by AFD ablation at 20°C. Bubble size is proportional to gene-set enrichment (observed/expected). Gene sets with significantly more or fewer genes than expected (p < 0.001, cell chi-square test) are colored red and blue, respectively; gene sets of the expected size (p > 0.05) are colored gray. (E, F) Gene Ontology (GO) term enrichment analysis. (E) Biological processes associated with the set of 508 upregulated genes (red bubbles) and the set of 629 downregulated genes (blue bubbles) with a statistically significant and greater than twofold change in expression in wild-type nematodes grown at 25°C relative to those grown at 20°C. (F) Biological processes associated with the set of 2001 upregulated genes (red bubbles) and the set of 1832 downregulated genes (blue bubbles) with a statistically significant and greater than twofold change in expression in AFD-ablated nematodes grown at 20°C relative to wild-type (unablated) nematodes grown at 20°C. Bubble size is proportional to the statistical significance [−log10(p value)] of enrichment. Biological processes that were induced or repressed by both interventions are bolded and shaded with darker red and blue colors, respectively. (G) Average changes in expression and 95% confidence intervals induced by growth at 25°C and AFD ablation at 20°C within intervals in the genomic region encoding the three C. elegans catalase genes. Gene models show the positions and splicing pattern of each catalase gene, intervals with 100% nucleotide identity are shown in orange (ctl-1 and ctl-2) and green (ctl-1 and ctl-3), and unique intervals are show in gray (ctl-2) and purple (ctl-3). The asterisks mark gene regions with significant fold-change in expression: **p < 0.001 and *p < 0.025, otherwise ‘ns’ indicates p > 0.1 (generalized linear model).
-
Figure 4—source data 1
mRNA sequencing (mRNA-seq) analysis data.
- https://cdn.elifesciences.org/articles/78941/elife-78941-fig4-data1-v2.xlsx
-
Figure 4—source data 2
mRNA sequencing (mRNA-seq) analysis data for the genomic region of the three catalase genes.
- https://cdn.elifesciences.org/articles/78941/elife-78941-fig4-data2-v2.xlsx
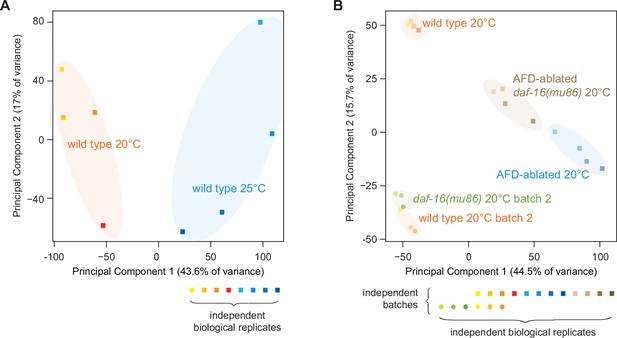
Principal component analysis (PCA) of the sequenced samples.
(A) PCA of the sequenced samples of wild-type nematodes grown at 25 and 20°C. (B) PCA of the sequenced samples of wild-type, daf-16(mu86) null mutants, AFD-ablated nematodes, and AFD-ablated daf-16(mu86) null mutants grown at 20°C.
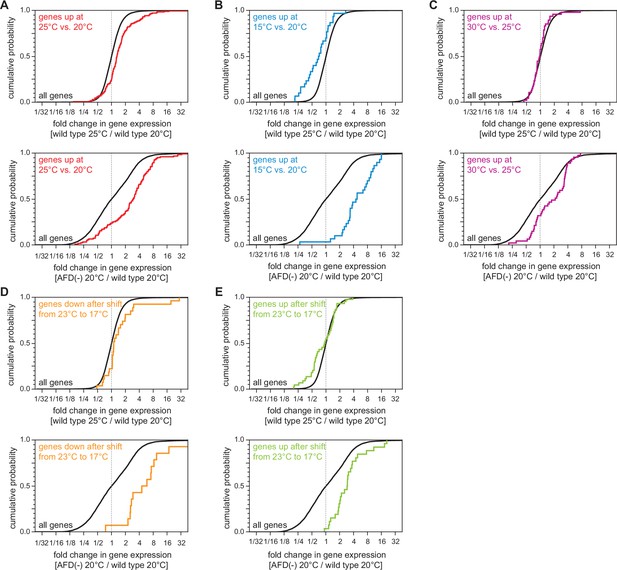
The AFD sensory neurons influence responses to noxious heat, high cultivation temperature, and low temperature.
(A–E) Effect on the expression of temperature-regulated gene sets of (top panels) growth at 25°C relative to growth at 20°C in wild-type nematodes and of (bottom panels) AFD ablation in nematodes grown at 20°C relative to wild-type (unablated) nematodes grown at 20°C: (A) genes up at 25 vs. 20°C (Gómez-Orte et al., 2018); (B) genes up at 15 vs. 20°C (Gómez-Orte et al., 2018); (C) genes up at 30 vs. 25°C (McCarroll et al., 2004); (D) genes down after shift from 23 to 17°C (Sugi et al., 2011); (E) genes up after shift from 23 to 17°C (Sugi et al., 2011). Statistical analyses for panels (A–E) are in Supplementary file 3.
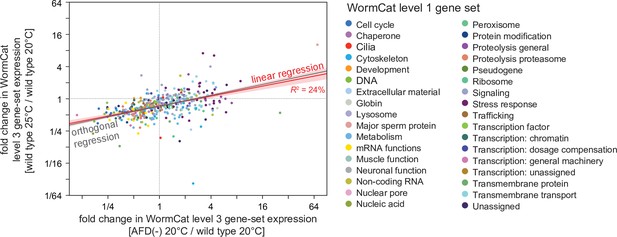
High cultivation temperature and AFD ablation induce positively correlated changes in the expression of gene sets affecting similar biological processes.
Growth at 25°C and AFD ablation at 20°C induced correlated changes in the average gene-expression level of gene sets affecting similar biological processes based on WormCat ‘level 3’ nested categories (Higgins et al., 2022; Holdorf et al., 2020). Gene sets are colored based on their WormCat ‘level 1’ categories. Linear regression fit is shown as a red line flanked by a red area marking the 95% confidence interval of the fit. The orthogonal regression fit (gray line) makes no assumptions about the dependence or independence of the variables.
-
Figure 4—figure supplement 3—source data 1
WormCat analysis data.
- https://cdn.elifesciences.org/articles/78941/elife-78941-fig4-figsupp3-data1-v2.xlsx
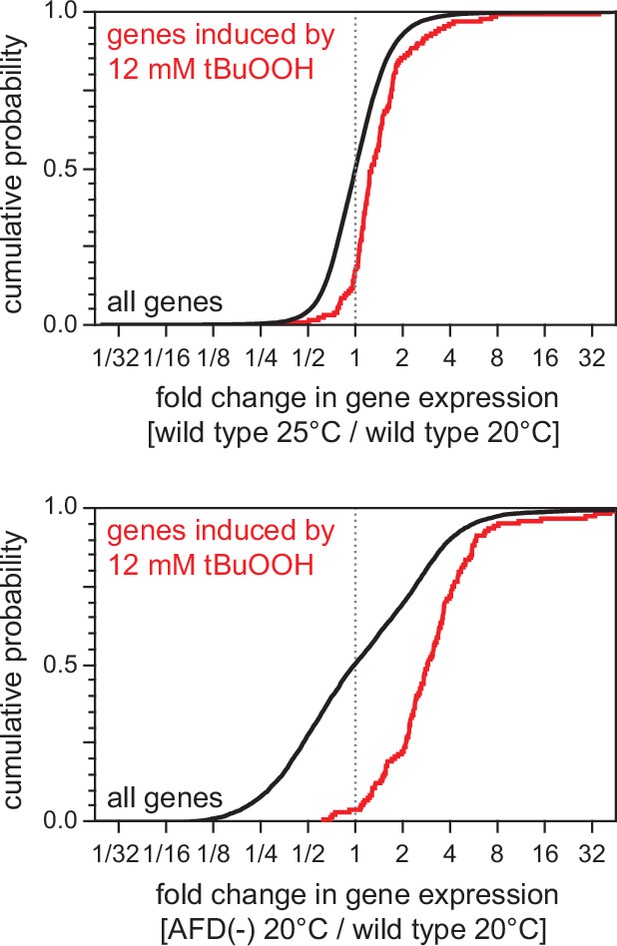
High cultivation temperature and AFD ablation pre-induce genes induced by tert-butyl hydroperoxide.
Effect on the expression of genes induced by 12 mM tert-butyl hydroperoxide (tBuOOH) (Oliveira et al., 2009) of (top panel) growth at 25°C relative to growth at 20°C in wild-type nematodes and of (bottom panel) AFD ablation in nematodes grown at 20°C relative to wild-type (unablated) nematodes grown at 20°C. Statistical analysis is in Supplementary file 3.
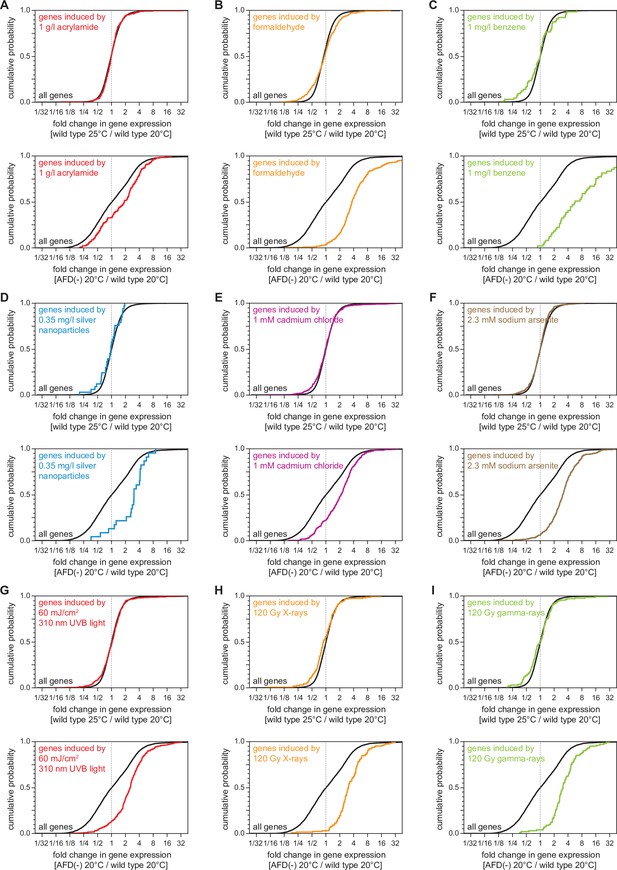
AFD ablation, but not high cultivation temperature, pre-induces genes induced by toxic organic compounds, toxic metals, and radiation.
(A–I) Effect on the expression of genes induced by toxic organic compounds, toxic metals, and radiation of (top panels) growth at 25°C relative to growth at 20°C in wild-type nematodes and of (bottom panels) AFD ablation in nematodes grown at 20°C relative to wild-type (unablated) nematodes grown at 20°C: (A) genes induced by 1 g/l acrylamide (Lewis et al., 2009); (B) genes induced by formaldehyde (Yang et al., 2016); (C) genes induced by 1 mg/l benzene (Eom et al., 2014); (D) genes induced by 0.35 mg/l silver nanoparticles (Starnes et al., 2016); (E) genes induced by 1 mM cadmium chloride (Huffman et al., 2004); (F) genes induced by 2.3 mM sodium arsenite (Sahu et al., 2013); (G) genes induced by 60 mJ/cm2 310 nm UVB light (Mueller et al., 2014); (H) genes induced by 120 Gy X-rays (Greiss et al., 2008); (I) genes induced by 120 Gy gamma rays (Greiss et al., 2008). Statistical analyses for panels (A–I) are in Supplementary file 3.
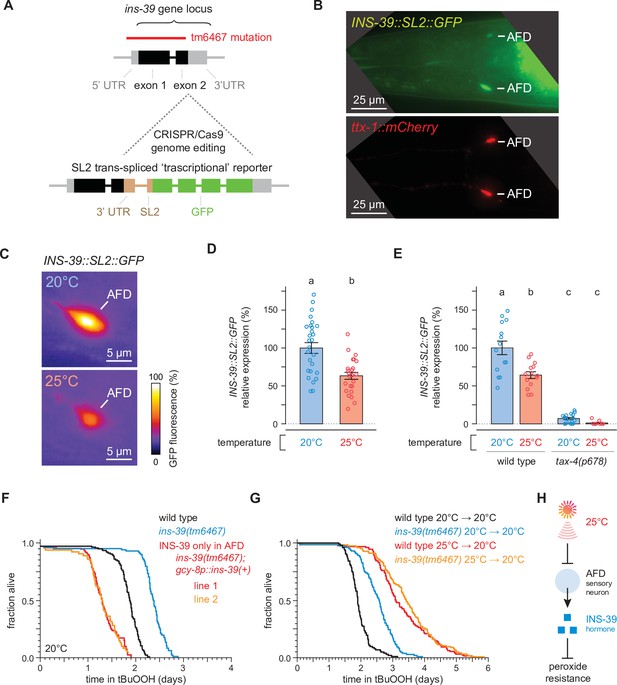
The high temperature-repressed INS-39 insulin/IGF1 hormone from the AFD sensory neurons lowers the nematode’s peroxide resistance.
(A) Schematic of the ins-39 gene locus showing the CRISPR/Cas9 genome editing strategy used to engineer the ins-39(oy167[ins-39::SL2::GFP]) ‘transcriptional’ reporter. The red line denotes the location of the ins-39(tm6467) deletion. (B) Example animal co-expressing the ins-39(oy167[ins-39::SL2::GFP]) reporter (top panel) and the AFD-specific reporter Ex[ttx-1p::TagRFP] (bottom panel). The head region is shown and only the AFD neurons are detected. Lines indicate the AFD soma. Scale bar = 25 µm. (C) Representative images of the expression of the ins-39(oy167[ins-39::SL2::GFP]) reporter in nematodes grown at 20°C (top panel) and 25°C (bottom panel) in one of the bilateral AFD neurons. Scale bar = 5 µm. (D, E) Quantification of the expression of the ins-39(oy167[ins-39::SL2::GFP]) reporter. (D) Reporter expression was lower in nematodes grown at 20°C than at 25°C. Data are represented as mean ± s.e.m. Groups labeled with different letters exhibited significant differences (n ≥ 25 in both groups, p < 0.0001, analysis of variance [ANOVA]). (E) Reporter expression was nearly abolished in tax-4(p678) mutants. Data are represented as mean ± s.e.m. Groups labeled with different letters exhibited significant differences (n ≥ 10 in each group, p < 0.0001, Tukey HSD test) otherwise (p > 0.05). (F) Peroxide resistance of wild-type, ins-39(tm6467), and ins-39(tm6467) with ins-39(+) reintroduced with the AFD-specific gcy-8 promoter in nematodes grown and assayed at 20°C. (G) The ins-39(tm6467) mutation increased peroxide resistance in nematodes grown and assayed at 20°C, but did not further increase peroxide resistance in nematodes grown at 25°C and assayed at 20°C. (H) Sensory perception of the cultivation temperature regulates the nematodes’ subsequent peroxide resistance. A high cultivation temperature lowers the expression of the AFD-specific INS-39 hormone, leading to the de-repression of the nematodes’ peroxide defenses. Statistical analysis for panels (F, G) is in Supplementary file 5.
-
Figure 5—source data 1
Survival data for panels F, G.
- https://cdn.elifesciences.org/articles/78941/elife-78941-fig5-data1-v2.xlsx
-
Figure 5—source data 2
Expression data for panels D, E.
- https://cdn.elifesciences.org/articles/78941/elife-78941-fig5-data2-v2.xlsx
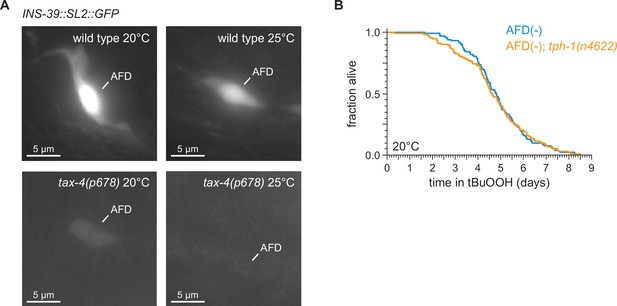
TAX-4 cyclic GMP-gated channels are required for ins-39 gene expression in the AFD sensory neurons.
(A) Representative images the expression of the ins-39(oy167[ins-39::SL2::GFP]) reporter in wild-type nematodes (top panels) and tax-4(p678) mutants (bottom panels), grown at 20°C (left panels) and 25°C (right panels) in one of the bilateral AFD neurons. Scale bar = 5 µm. (B) tph-1(n4622) did not affect the increased peroxide resistance of AFD-ablated nematodes at 20°C. Statistical analysis for panel (B) is in Supplementary file 5.
-
Figure 5—figure supplement 1—source data 1
Survival data for panel B.
- https://cdn.elifesciences.org/articles/78941/elife-78941-fig5-figsupp1-data1-v2.xlsx
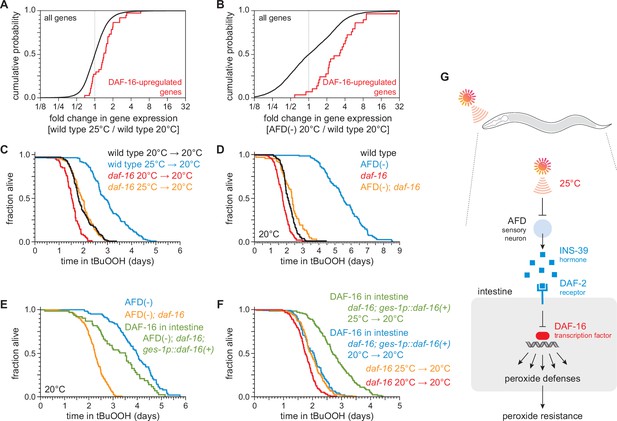
DAF-16/FOXO functions in the intestine to increase the nematode’s peroxide resistance in response to temperature-dependent signals from the AFD sensory neurons.
Genes directly upregulated by DAF-16 (Kumar et al., 2015) had higher expression (A) in nematodes grown at 25°C than in nematodes grown at 20°C and (B) in AFD-ablated nematodes grown at 20°C than in wild-type (unablated) nematodes grown at 20°C. daf-16(mu86) suppressed most of the increased peroxide resistance of (C) nematodes grown at 25°C and assayed at 20°C and (D) AFD-ablated nematodes grown at 20°C. (E) Peroxide resistance of AFD-ablated nematodes expressing daf-16(+) only in the intestine, AFD-ablated daf-16(mu86) controls, and AFD-ablated nematodes for reference. Nematodes were grown and assayed at 20°C. (F) Peroxide resistance of transgenic nematodes expressing daf-16(+) only in the intestine, and daf-16(mu86) controls. Nematodes were grown at the indicated temperatures and assayed at 20°C. (G) The AFD sensory neurons repress the expression of H2O2-protection services in the nematode’s intestine via insulin/IGF1 signaling. AFD expresses high levels of INS-39 at the lower cultivation temperature (20°C), leading to repression of the CTL-1 and CTL-2 H2O2-degrading catalases and of other peroxide defenses. At the higher cultivation temperature (25°C), AFD lowers INS-39 expression, de-repressing the DAF-16/FOXO factor that increases the expression of peroxide defenses in the intestine. Statistical analyses for panels (A, B) are in Supplementary file 3 and statistical analyses for panels (C–F) are in Supplementary file 7.
-
Figure 6—source data 1
Survival data for panels C–F.
- https://cdn.elifesciences.org/articles/78941/elife-78941-fig6-data1-v2.xlsx
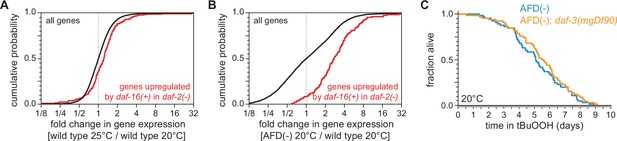
The AFD sensory neurons repress the expression of genes induced by DAF-16/FOXO.
Genes upregulated in a daf-16-dependent manner in daf-2(−) mutants (Murphy et al., 2003) had higher expression (A) in nematodes grown at 25°C than in nematodes grown at 20°C and (B) in AFD-ablated nematodes grown at 20°C than in wild-type (unablated) nematodes grown at 20°C. (C) daf-3(mgDf90) did not affect the increased peroxide resistance of AFD-ablated nematodes at 20°C. Statistical analyses for panels (A, B) are in Supplementary file 3 and statistical analysis for panel (C) is in Supplementary file 7.
-
Figure 6—figure supplement 1—source data 1
Survival data for panel C.
- https://cdn.elifesciences.org/articles/78941/elife-78941-fig6-figsupp1-data1-v2.xlsx
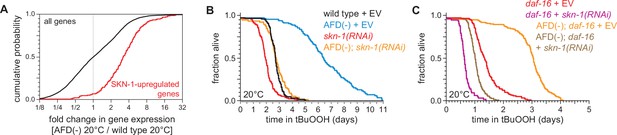
SKN-1/NRF and DAF-16/FOXO collaborate to increase the nematodes’ peroxide resistance in response to signals from the AFD sensory neurons.
(A) Genes upregulated by skn-1(+) in wild-type nematodes (Oliveira et al., 2009) had higher expression in AFD-ablated nematodes grown at 20°C than in wild-type (unablated) nematodes grown at 20°C. (B) skn-1(RNAi) suppressed most of the increased peroxide resistance of AFD-ablated nematodes grown at 20°C. Control RNAi consisted of feeding the nematodes the same bacteria but with the empty vector (EV) plasmid pL4440 instead of a plasmid targeting skn-1. (C) skn-1(RNAi) lowered peroxide resistance to a greater extent in AFD-ablated daf-16(mu86) mutants at 20°C than in (unablated) daf-16(mu86) mutants at 20°C. Statistical analysis for panel (A) is in Supplementary file 3 and statistical analyses for panels (B, C) are in Supplementary file 8.
-
Figure 7—source data 1
Survival data for panels B, C.
- https://cdn.elifesciences.org/articles/78941/elife-78941-fig7-data1-v2.xlsx
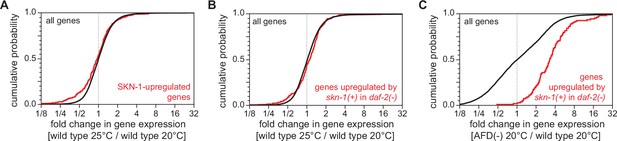
The AFD sensory neurons repress the expression of genes induced by SKN-1/NRF.
Genes upregulated by skn-1(+) in (A) wild-type nematodes (Oliveira et al., 2009) and (B) daf-2 loss-of-function mutants (Ewald et al., 2015) did not have higher expression in nematodes grown at 25°C than in nematodes grown at 20°C. (C) Genes upregulated by skn-1(+) in daf-2 loss-of-function mutants (Ewald et al., 2015) had higher expression in AFD-ablated nematodes grown at 20°C than in wild-type (unablated) nematodes grown at 20°C. Statistical analyses for panels (A–C) are in Supplementary file 3.
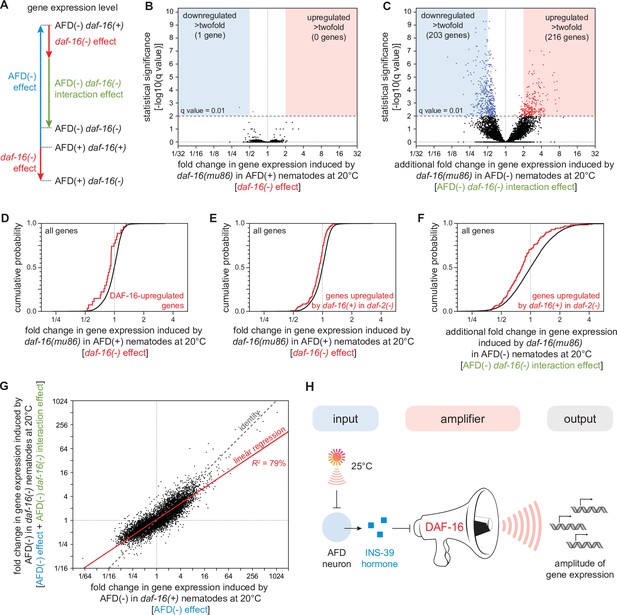
DAF-16/FOXO potentiates the changes in gene expression induced by the AFD sensory neurons.
(A) We performed mRNA sequencing (mRNA-seq) on wild-type [AFD(+) daf-16(+)], daf-16(mu86) null mutants [AFD(+) daf-16(−)], AFD-ablated nematodes [AFD(−) daf-16(+)], and AFD-ablated daf-16(mu86) null mutants [AFD(−) daf-16(−)] grown at 20°C, and used an epistasis model to quantify the extent to which AFD ablation and daf-16 mutation affected the expression of each gene, relative to wild-type, in terms of the independent effects induced by AFD ablation (blue arrow) and by lack daf-16 gene function (red arrow), and the additional effect induced by the interaction between AFD ablation and lack daf-16 gene function (green arrow). Volcano plots showing the level and statistical significance of (B) the changes in gene expression induced by lack daf-16 gene function in unablated nematodes at 20°C and (C) the additional changes in gene expression induced by lack daf-16 gene function in AFD-ablated nematodes at 20°C. Genes up- and downregulated significantly (q value < 0.01) are shown in red and blue, respectively. (D, E) Effect of lack daf-16 gene function in unablated nematodes at 20°C on the expression of (C) genes directly upregulated by DAF-16 (Kumar et al., 2015) and (D) genes upregulated in a daf-16-dependent manner in daf-2(−) mutants (Murphy et al., 2003). (F) Effect of the additional changes in gene expression induced by lack daf-16 gene function in AFD-ablated nematodes at 20°C on the expression of genes upregulated in a daf-16-dependent manner in daf-2(−) mutants (Murphy et al., 2003). (G) The effect of AFD ablation on gene expression at 20°C was systematically smaller in daf-16(mu86) mutants (y-axis) than in daf-16(+) nematodes (x-axis). Linear regression fit is shown as a red line flanked by a red area marking the 95% confidence interval of the fit. (H) The DAF-16/FOXO transcription factor amplifies the changes in gene expression induced by AFD ablation. This means that DAF-16 determines the gene-expression responsiveness, but not the response to signals from the AFD sensory neurons. Statistical analyses for panels (D–F) are in Supplementary file 3.
-
Figure 8—source data 1
mRNA sequencing (mRNA-seq) analysis data.
- https://cdn.elifesciences.org/articles/78941/elife-78941-fig8-data1-v2.xlsx
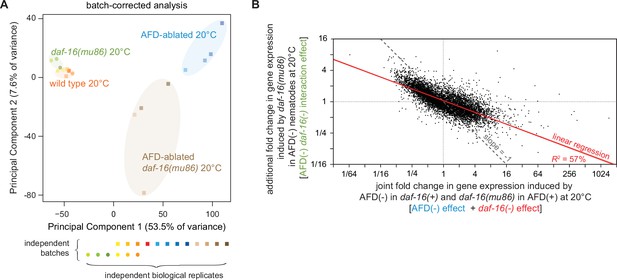
Gene expression is regulated by the interaction of the AFD sensory neurons and DAF-16/FOXO.
(A) Principal component analysis (PCA) of the batch-corrected sequenced samples of wild-type, daf-16(mu86) null mutants, AFD-ablated nematodes, and AFD-ablated daf-16(mu86) null mutants grown at 20°C. See Figure 4—figure supplement 1A for the PCA before batch correction. (B) The extent to which lack daf-16 function had additional effects on gene expression when the AFD neurons were ablated was negatively correlated with the level of gene expression predicted if AFD and DAF-16 acted independently. Linear regression fit is shown as a red line flanked by a red area marking the 95% confidence interval of the fit. The slope and intercept of that line would have been 0, if AFD and DAF-16 had acted independently to influence fold changes in gene expression at the transcriptome level (Angeles-Albores et al., 2018).
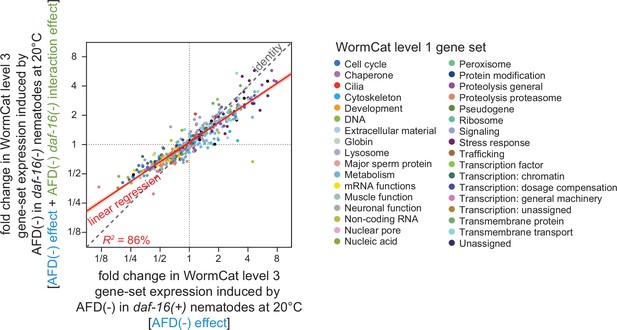
DAF-16/FOXO potentiates the changes in gene-set expression induced by the AFD sensory neurons.
The effect of AFD ablation at 20°C on the average gene-expression level of gene sets affecting similar biological processes based on WormCat ‘level 3’ nested categories (Higgins et al., 2022; Holdorf et al., 2020) was systematically smaller in daf-16(mu86) mutants (y-axis) than in daf-16(+) nematodes (x-axis). Gene sets are colored based on their WormCat ‘level 1’ categories. Linear regression fit is shown as a red line flanked by a red area marking the 95% confidence interval of the fit.
-
Figure 8—figure supplement 2—source data 1
WormCat analysis data.
- https://cdn.elifesciences.org/articles/78941/elife-78941-fig8-figsupp2-data1-v2.xlsx
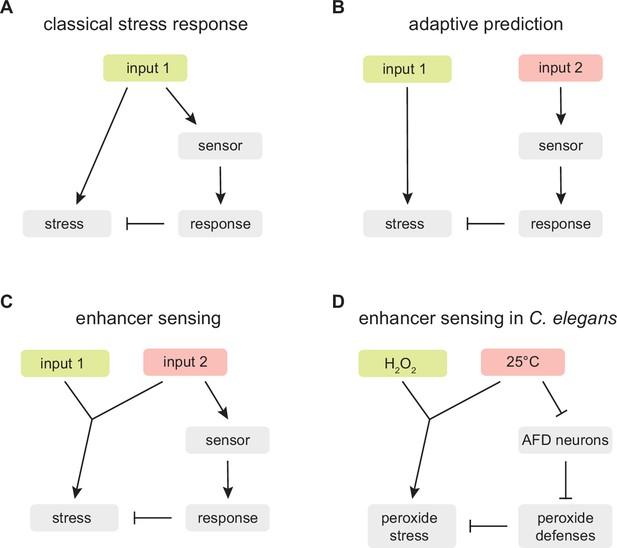
An enhancer sensing strategy enables C.elegans to assess faithfully the threat of hydrogen peroxide using temperature information.
(A) Classical stress response: the strategy provides faithful information about the threat the organism faces because the response that enables the organism to cope with the stress induced by input 1 is coupled to the perception of input 1. (B) Adaptive prediction: the strategy provides a guess (whose predictive value matches the co-occurrence of inputs 1 and 2 in the ecological setting of the organism) but not necessarily faithful information about the threat the organism faces, because input 2 does not induce (nor affect the capacity of input 1 to induce) the stress that the organism attempts to cope with by inducing a response to input 2. (C) Enhancer sensing: the strategy provides faithful information about the threat the organism faces because the capacity of input 1 to induce a stress is modulated by input 2 and, therefore, perception of either input provides information about the threat posed by the interaction of those inputs. (D) The nematode C. elegans uses an enhancer sensing strategy that couples the de-repression of specific H2O2 defenses to the sensory perception of high temperature, an inherent enhancer of the reactivity of H2O2.
Additional files
-
Supplementary file 1
Statistical analysis for Figure 2 and Figure 2—figure supplement 1.
- https://cdn.elifesciences.org/articles/78941/elife-78941-supp1-v2.xlsx
-
Supplementary file 2
Statistical analysis for Figure 3 and Figure 3—figure supplement 1.
- https://cdn.elifesciences.org/articles/78941/elife-78941-supp2-v2.xlsx
-
Supplementary file 3
Statistical analysis of gene-set expression for Figures 6—8; Figure 4—figure supplements 2 and 4, and 5; Figure 6—figure supplement 1; and Figure 7—figure supplement 1.
- https://cdn.elifesciences.org/articles/78941/elife-78941-supp3-v2.xlsx
-
Supplementary file 4
Gene Ontology analysis for Figure 4.
- https://cdn.elifesciences.org/articles/78941/elife-78941-supp4-v2.xlsx
-
Supplementary file 5
Statistical analysis for Figure 5 and Figure 5—figure supplement 1.
- https://cdn.elifesciences.org/articles/78941/elife-78941-supp5-v2.xlsx
-
Supplementary file 6
Regulation of DAF-16 target genes among genes up- and down-regulated significantly (q value <0.01) by growth at 25°C and by AFD ablation at 20°C.
- https://cdn.elifesciences.org/articles/78941/elife-78941-supp6-v2.xlsx
-
Supplementary file 7
Statistical analysis for Figure 6 and Figure 6—figure supplement 1.
- https://cdn.elifesciences.org/articles/78941/elife-78941-supp7-v2.xlsx
-
Supplementary file 8
Statistical analysis for Figure 7.
- https://cdn.elifesciences.org/articles/78941/elife-78941-supp8-v2.xlsx
-
Supplementary file 9
Bacterial strains.
- https://cdn.elifesciences.org/articles/78941/elife-78941-supp9-v2.xlsx
-
Supplementary file 10
Nematode strains.
- https://cdn.elifesciences.org/articles/78941/elife-78941-supp10-v2.xlsx
-
Supplementary file 11
PCR genotyping primers and phenotypes used for strain construction.
- https://cdn.elifesciences.org/articles/78941/elife-78941-supp11-v2.xlsx
-
MDAR checklist
- https://cdn.elifesciences.org/articles/78941/elife-78941-mdarchecklist1-v2.docx





