CRMP4-mediated fornix development involves Semaphorin-3E signaling pathway
Figures
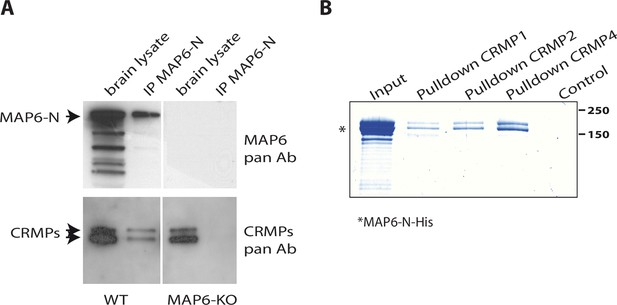
Identification of collapsin response mediator protein 1 (CRMP1), -2, and -4 as microtubule-associated protein 6 (MAP6)-binding partners.
(A) Immunoprecipitation of endogenous CRMPs from adult mouse brain using mAb-175. CRMPs were revealed using anti-pan-CRMP antibody and MAP6 using anti-pan-MAP6 antibody. Lysate from MAP6-KO mouse brain was used as control. (B) Coomassie gel of pull-down experiments. Control Sepharose beads (control lane) or CNBr-coupled CRMPs-Sepharose beads were incubated with purified MAP6-N-His protein. Input lane corresponds to the total amount of MAP6-N-His (2 µg) incubated with the CRMPs-Sepharose beads (5 µL). Molecular weights are indicated in kDa.
-
Figure 1—source data 1
Extended view of panels A and B.
- https://cdn.elifesciences.org/articles/70361/elife-70361-fig1-data1-v5.zip
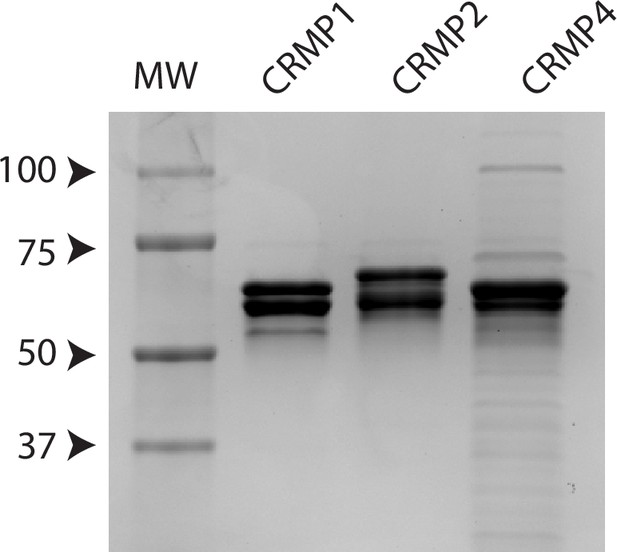
Recombinant collapsin response mediator protein family (CRMP) proteins.
Coomassie blue staining of purified recombinant CRMPs (2 µg for each sample).
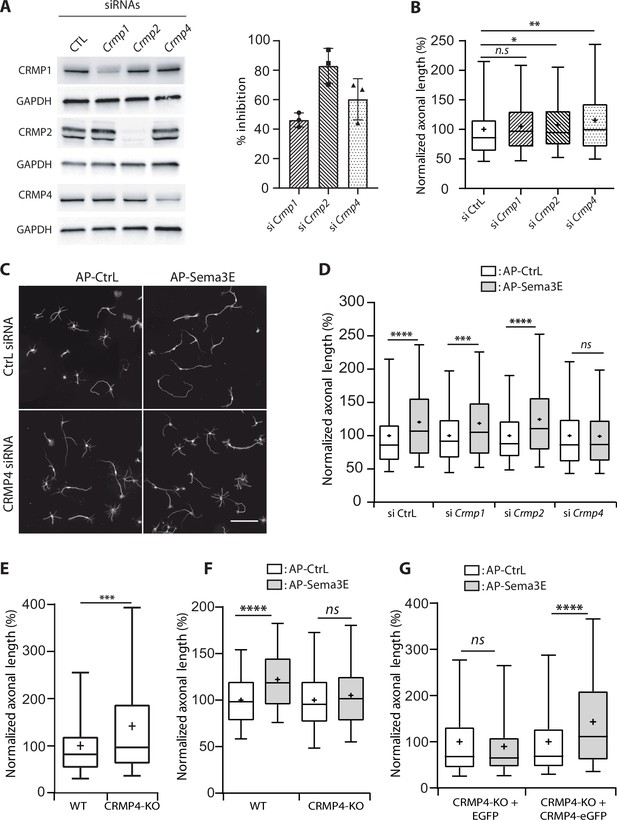
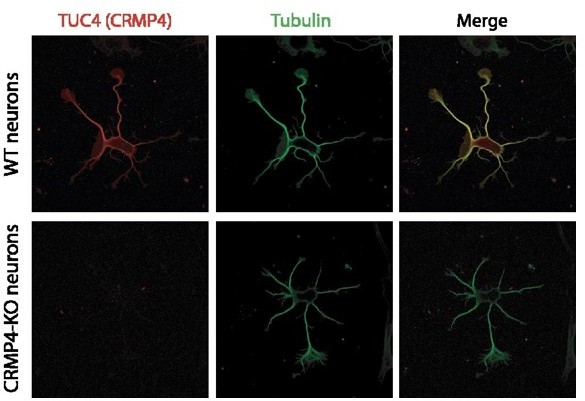
Effect of collapsin response mediator protein family (CRMP) downregulation on Semaphorin-3E (Sema3E) growth-promoting activity.
(A) Western blot of crude extracts from cultured subicular neurons treated for 48 hr with siRNA for CRMPs or control siRNA, and blotted with CRMP and GAPDH antibodies. Right panel: quantification of siRNA efficiency, values are expressed as percent inhibition compared to control siRNA (n = 3 independent experiments). (B) Axonal length measured in cultured subicular neurons treated for 48 hr with control siRNAs and CRMP siRNAs (20 nM). Values were normalized to 100% for control siRNAs. (C) Representative images of subicular neurons electroporated with control or CRMP4 siRNA and cultured in the absence (AP-CtrL) or presence of 5 nM Sema3E (AP-Sema3E) for 48 hr. To measure axonal length, neurons were immunolabeled with anti-α-tubulin. Scale bar, 100 µm. (D) Quantification of the relative axonal length of cultured subicular neurons treated for 48 hr with 20 nM of control or CRMP siRNAs in the absence (AP-CtrL) or presence of 5 nM Sema3E (AP-Sema3E). Values were normalized to 100% for the AP control condition (AP-CtrL). (E) Quantification of relative axonal length in cultured wild-type (WT) and CRMP4-KO subicular neurons. Values were normalized to 100% for WT neurons. (F) Quantification of the relative axonal length of cultured WT and CRMP4-KO subicular neurons in the absence (AP-CtrL) or presence of 5 nM Sema3E (AP-Sema3E). Values were normalized to 100% for the AP control (AP-CtrL) condition. (G) Quantification of the relative axonal length of cultured CRMP4-KO subicular neurons electroporated with control plasmid coding for eGFP or for eGFP-tagged CRMP4 in the absence (AP-CtrL) or presence of 5 nM Sema3E (AP-Sema3E). Values were normalized to 100% for the AP control condition. In B, D, E, F, and G, crosses represent the mean, black bars represent the median, boxes represent the 25th and 75th percentiles, and the whiskers represent the 5th and 95th percentiles of values (72 neurons for each condition) from three independent cultures. Kruskal-Wallis non-parametric test followed by Dunn’s multiple comparisons, ****p < 0.0001, ***p < 0.001, **p < 0.01, *p < 0.05 and n.s. p > 0.05.
-
Figure 2—source data 1
Extended view of panel A and raw data of A, B, D-G.
- https://cdn.elifesciences.org/articles/70361/elife-70361-fig2-data1-v5.zip
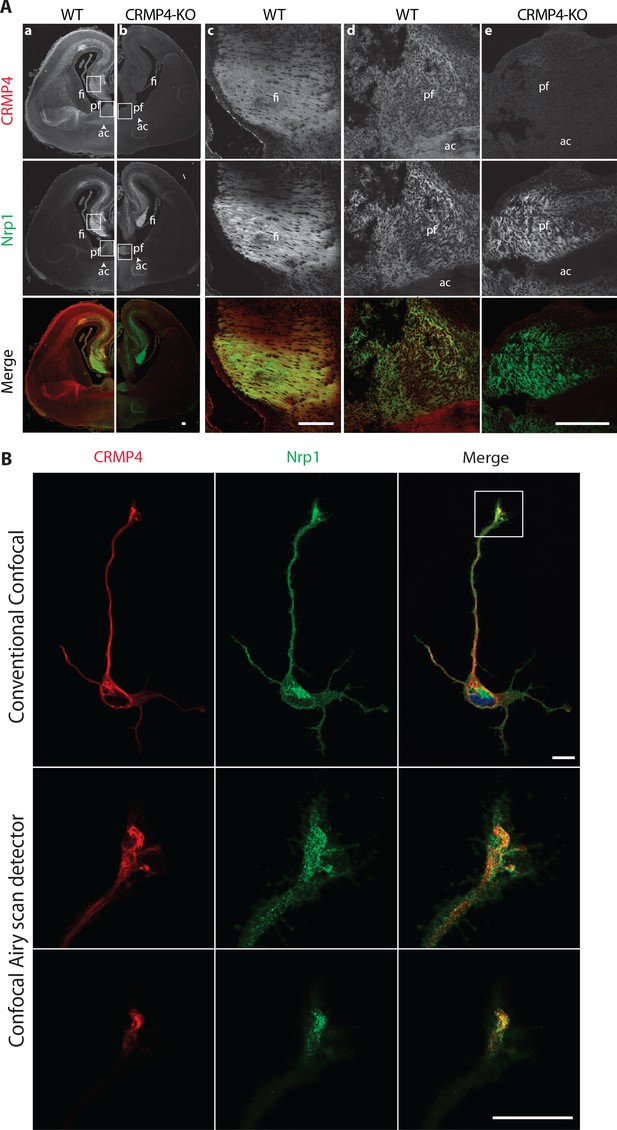
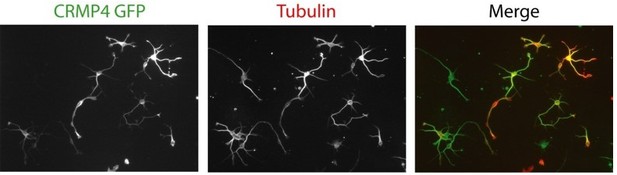
Collapsin response mediator protein 4 (CRMP4) expression in subicular neurons and fornix projections.
(A) Coronal sections of embryonic day 17.5 (E17.5) wild-type (WT) (columns a, c, d) and CRMP4-KO (columns b, e) brain were immunolabeled with anti-CRMP4 and anti-Nrp1 antibodies. White squares (columns a and b) indicate the positions of fields of view shown in columns c–e. Abbreviations: ac: anterior commissure, fi: fimbria; pf: post-commissural fornix. Scale bars, 100 µm. (B) Cultured subicular neurons at 2 days in vitro (DIV) were immunolabeled with anti-CRMP4 and anti-Nrp1 antibodies. The conventional confocal plane shows the entire neuron. Images from the confocal Airyscan detail the growth cone on two serial acquisition planes. Scale bars, 10 µm.
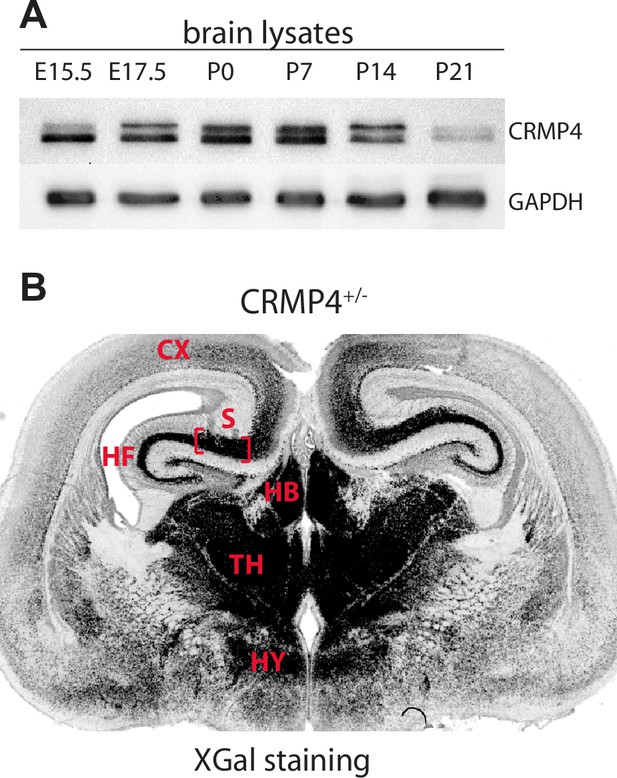
CRMP4 protein and gene expression during brain development.
(A) Western blot showing CRMP4 expression levels during embryonic and postnatal development of the mouse brain. CRMP4 and GAPDH proteins were analyzed by western blot using TUC4 and GAPDH antibodies, respectively. (B) CRMP4 gene expression was revealed by X-Gal staining on a coronal brain section from CRMP4+/- mice at E17.5. The subiculum (flanked with red brackets) presents strong LacZ activity. Abbreviations: CX: cortex, HB: habenula, HF: hippocampal formation, HY: hypothalamus, S: subiculum, and TH: thalamus.
-
Figure 3—figure supplement 1—source data 1
Extended view of panel A.
- https://cdn.elifesciences.org/articles/70361/elife-70361-fig3-figsupp1-data1-v5.zip
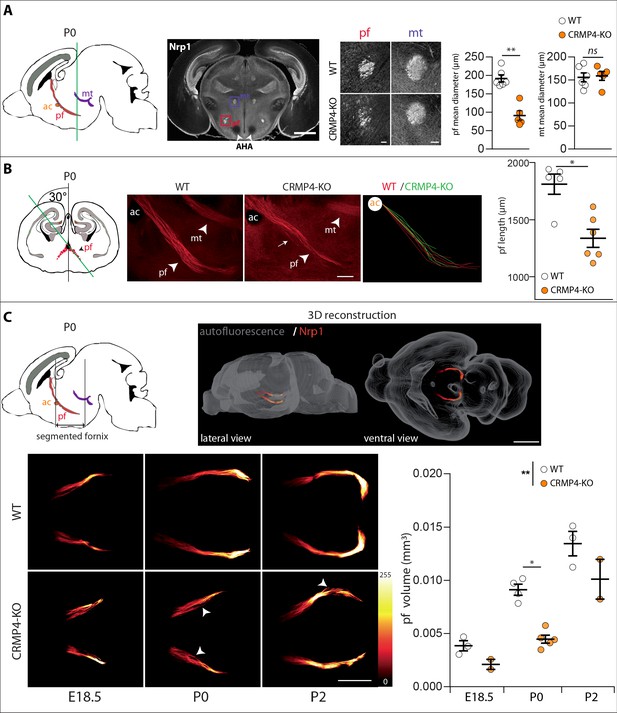
Effect of collapsin response mediator protein 4 knockout (CRMP4-KO) on post-commissural fornix formation.
(A) From left to right: The sagittal diagram shows the projection stacks for the post-commissural fornix (pf; red) and mammillo-thalamic tract (mt, purple); the green line indicates the level of the coronal section. Representative coronal section corresponding to the onset of the anterior part of the anterior hypothalamic area (AHA) in postnatal day 0 (P0) wild-type (WT) and CRMP4-KO brains immunolabeled with anti-neuropilin-1 (Nrp1) antibody. Fornix and mammillo-thalamic tract were squared. Scale bar, 1 mm. Higher-magnification images show representative post-commissural and mammillo-thalamic tracts in CRMP4-KO and WT brains after Nrp1 immunolabeling. Scale bars, 100 µm. Dot plots show quantifications of both fornix and mammillo-thalamic tract diameters. Mean ± s.e.m. WT n = 6, KO n = 5, Mann-Whitney test, *p < 0.001. (B) From left to right: The coronal diagram shows the projection stack of post-commissural fornix (red) on a coronal plane. The green line indicates the plane of the sagittal sections. Representative sagittal sections with a cutting angle of 30° for P0 WT and CRMP4-KO brains were immunolabeled with anti-Nrp1 antibody. The lengths of post-commissural fornices were measured using ImageJ, taking the anterior commissure as the starting point. All traces measured from WT (n = 5; red) and CRMP4-KO (n = 6; green) were superimposed and are shown in the right panel. The dot plot shows quantifications of post-commissural fornices. Scale bar, 250 µm. Mean ± s.e.m. Mann-Whitney test, *p < 0.05. (C) The sagittal diagram shows the projection stack of the post-commissural fornix (pf, in red) and black lanes indicate the segmented portion of the fornix. The anterior commissure (ac, in orange) was used as the starting point to segment the fornix (upper left diagram). A representative P2 segmented fornix is shown in 3D reconstruction (Nrp1 in red) in the whole brain (autofluorescence in grey), illustrated in lateral view and ventral view (upper right panel). Reconstructions were produced using the ImageJ 3D Viewer plugin. Scale bar, 1 mm. Representative ventral z projections of WT and CRMP4-KO fornices at embryonic day 18.5 (E18.5), P0, and P2 are presented (bottom left panels). The fluorescence intensity of the segmented fornix is represented by a red-to-white color scale. White arrowheads point to axon bundles splitting off from the fornix. Scale bar, 500 µm. Histogram shows quantifications of the segmented post-commissural fornix volume at E18.5 (WT = 3; KO = 2), P0 (WT = 4, KO = 5) and P2 (WT = 3; KO = 2) for WT and CRMP4-KO mice. Bars indicate the mean volume ± s.e.m. Kruskal-Wallis non-parametric test followed by Dunn’s multiple comparisons and one-way ANOVA to compare WT vs. KO, *p < 0.05, **p < 0.01.
-
Figure 4—source data 1
Raw data of panel A-C.
- https://cdn.elifesciences.org/articles/70361/elife-70361-fig4-data1-v5.xlsx
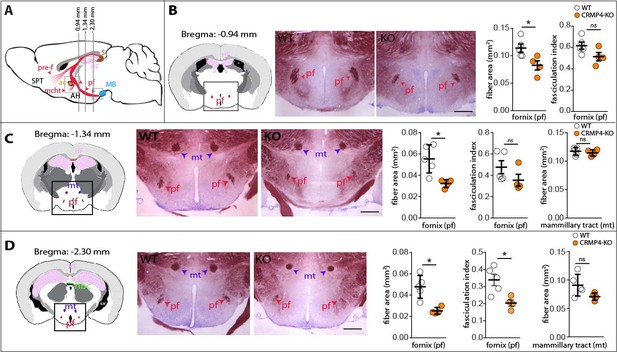
Deletion of collapsin response mediator protein 4 (CRMP4) affects fornix integrity in the adult brain.
(A) Schematic representation of the fornix tract (red region) on a sagittal diagram. Grey lines indicate the Bregma positions, in mm, of the coronal planes presented in B, C, and D. (B–D) Coronal brain sections from adult wild-type (WT) and CRMP4-knockout (KO) brains were stained with gold chloride. Diagrams of adult brain coronal slices illustrating the anatomical levels selected to measure surfaces covered by the fornix (pf) and mammillo-thalamic tract (mt). Square in the diagrams indicate the regions shown on the coronal sections stained with gold chloride. Histograms show quantifications of fiber areas and fasciculation index, mean ± s.e.m. WT = 5, KO = 4. Mann-Whitney test, *p < 0.05. Scale bar, 250 µm. Abbreviations: HF: hippocampal formation; MB: mammillary body; AH: anterior hypothalamus; SPT: septum; pre-f: pre-commissural fornix; pf: post-commissural fornix; mt: mammillo-thalamic tract; mcht, medial cortico-hypothalamic tract; ac: anterior commissure; S: subiculum.
-
Figure 5—source data 1
Raw data of panels A-D.
- https://cdn.elifesciences.org/articles/70361/elife-70361-fig5-data1-v5.xlsx
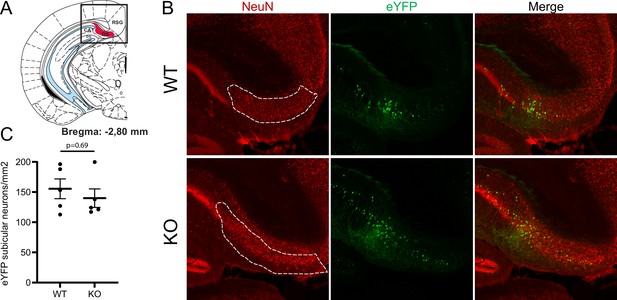
Comparison of the number of subicular neurons in wild-type (WT) and collapsin response mediator protein 4 (CRMP4) dorsal subicula.
Coronal sections of the subiculum from adult WT and CRMP4-knockout (KO) animals expressing the reporter eYFP in the subiculum. The dorsal subiculum shown in the diagram of Bregma –2.80 mm section (A) was delimited on the NeuN immunolabeling slice (B, dashed region of interest [ROI]). The density of eYFP-stained subicular neurons in WT and CRMP4-KO dorsal subicula was quantified, and the results are shown in (C). Difference between genotypes was not significant (n = 4 animals for each genotype).
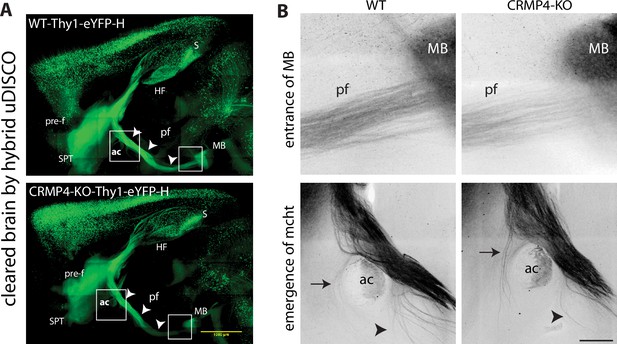
Visualization of the whole fornix system by uDISCO tissue clearing.
(A) Adult half-brains from wild-type (WT)- and CRMP4-KO-Thy1-eYFP-H mice were cleared by a hybrid uDISCO method (see Material and methods section). Representative broad panoramic views of z projections of WT and CRMP4-KO are shown. White squares indicate regions shown in B. Scale bar, 1 mm. Voxel size, x = 2.07, y = 2.07, z = 5.77 µm. (B) Higher magnifications of z projections show post-commissural fornix fibers (pf) entering mammillary bodies (MB) and mcht fibers emerging from the fornix. Arrows and arrowheads indicate the mcht fibers emerging from the anterior and posterior sides, respectively, of the anterior commissure (ac). Scale bar, 200 µm. Abbreviations: HF: hippocampal formation, MB: mammillary body; S: subiculum; SPT: septum; ac: anterior commissure; mcht: medial cortico-hypothalamic tract; pf: post-commissural fornix; and pre-f: pre-commissural fornix.
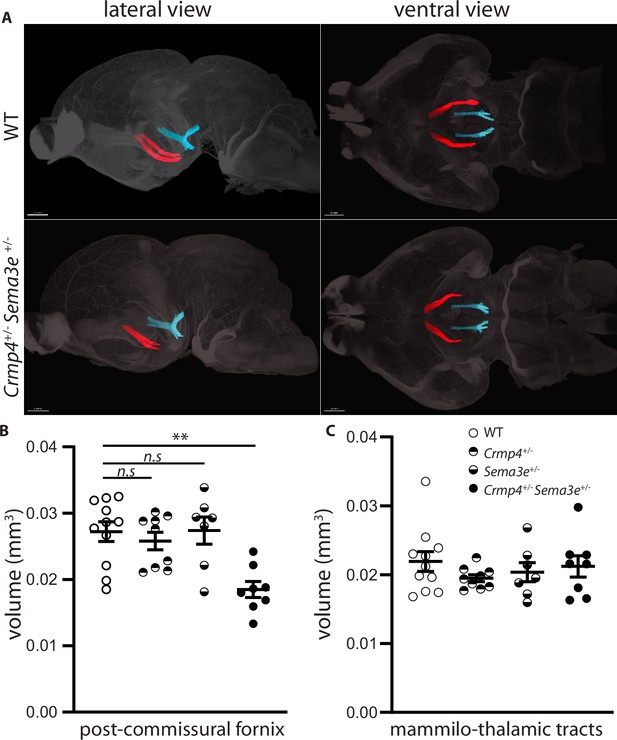
Post-commissural fornices are affected in collapsin response mediator protein 4 (Crmp4+/-)/Semaphorin-3E (Sema3e+/-) double heterozygotes.
(A) Postnatal day 0 (P0) segmented fornices (red) and mammillo-thalamic tracts (blue) 3D reconstructions in whole brains are illustrated in lateral view (left panel) and ventral view (right panel). Whole brains from single and double heterozygotes for Crmp4 and Sema3e and their wild-type (WT) littermates at P0 were immunolabeled with Nrp1 antibody and cleared according to the iDISCO protocol. Fornices and mammillo-thalamic fiber bundles were segmented using Imaris software. (B) Quantification of fornix (left panel) and mammillo-thalamic tract (right panel) volumes in P0 brains from WT (n = 11), Crmp4+/- (n = 9), Sema3e+/- (n = 7), and Crmp4+/-/Sema3e+/- (n = 8) mice. Voxel size: x = 3.02; y = 3.02; z = 3 µm; Bars indicate the mean volume ± s.e.m. Kruskal-Wallis non-parametric test followed by Dunn’s multiple comparisons, **p < 0.01, n.s. p > 0.05.
-
Figure 6—source data 1
Raw data of panel B.
- https://cdn.elifesciences.org/articles/70361/elife-70361-fig6-data1-v5.xlsx
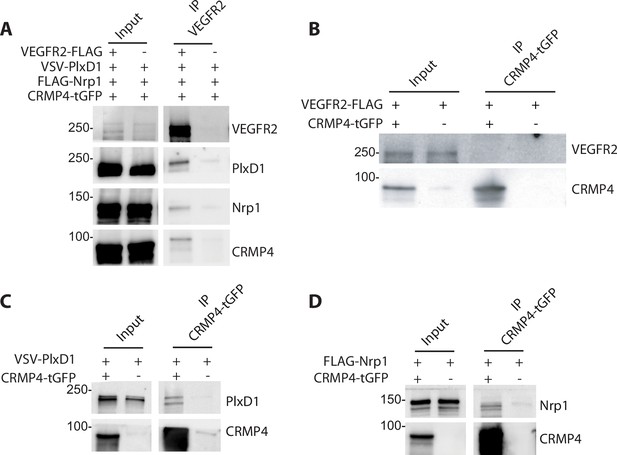
Collapsin response mediator protein 4 (CRMP4) forms complexes with Semaphorin-3E (Sema3E) receptors.
(A) Western blot showing co-immunoprecipitation of CRMP4 and Sema3E receptors. HEK293T/17 cells were transfected with plasmids encoding CRMP4-tGFP, VEGFR2-FLAG, VSV-PlxD1, and FLAG-Nrp1 proteins. Cells were lysed in buffer containing 0.5% Triton X-100, and immunoprecipitation was performed using a polyclonal anti-VEGFR2 antibody. (B–D) Western blot showing co-immunoprecipitation of CRMP4 with each individual Sema3E receptor. HEK293T/17 cells were transfected with plasmids encoding CRMP4-tGFP and with plasmids encoding VEGFR2-FLAG (B), VSV-PlxD1 (C), or FLAG-Nrp1 (D). Cells were lysed in buffer containing 0.5% Triton X-100 and immunoprecipitation was performed using a polyclonal anti-tGFP antibody.
-
Figure 7—source data 1
Extended view of panels A-D.
- https://cdn.elifesciences.org/articles/70361/elife-70361-fig7-data1-v5.zip
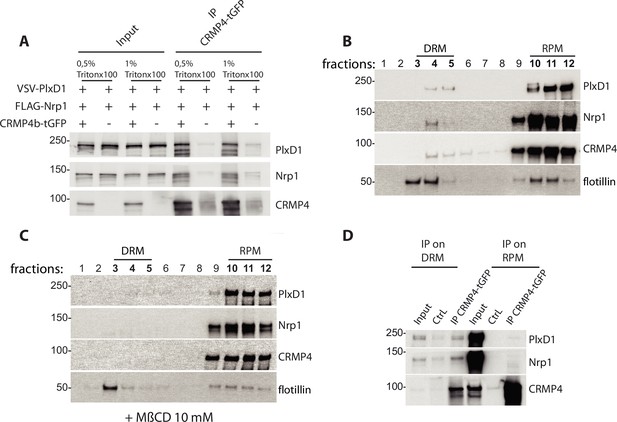
Collapsin response mediator protein 4 (CRMP4) interacts with the Semaphorin-3E (Sema3E) receptor complex in detergent-resistant membrane (DRM) fractions.
(A) HEK293T/17 cells were transfected with plasmids encoding CRMP4-tGFP, VSV-PlxD1, and FLAG-Nrp1 proteins. Cells were lysed in buffer containing 0.5% Triton X-100 (as used in Figure 7) or 1% Triton X-100. Immunoprecipitations were performed using a polyclonal anti-tGFP antibody. Proteins were analyzed by western blotting with PlxD1, Nrp1, and tGFP antibodies. (B and C) Sucrose-density gradient fractionation of lysates prepared with 1% Triton X-100 from HEK293T/17 cells transfected with plasmids encoding ectopic CRMP4-tGFP, VSV-PlxD1, and FLAG-Nrp1 proteins. HEK293T/17 cells were cultured in the absence (B) or presence (C) of 10 mM methyl-beta-cyclodextrin (MβCD) for 7 hr. Fractions were then analyzed by western blot. Ectopic proteins were assayed with PlxD1, Nrp1, and tGFP antibodies, and endogenous flotillin was detected with flotillin antibodies. (D) Western blot showing co-immunoprecipitations between CRMP4 and the Sema3E receptor complex from sucrose gradient fractions corresponding to DRM (3, 4, and 5) or to the remaining plasma membrane (RPM) (10, 11, and 12, see left panel in A), using a polyclonal anti-tGFP antibody. In control conditions (CtrL), no tGFP antibody was added to the pooled fractions. Equivalent volumes of pooled fraction (DRM and RPM) were loaded (input).
-
Figure 8—source data 1
Extended view of panels A-D.
- https://cdn.elifesciences.org/articles/70361/elife-70361-fig8-data1-v5.zip
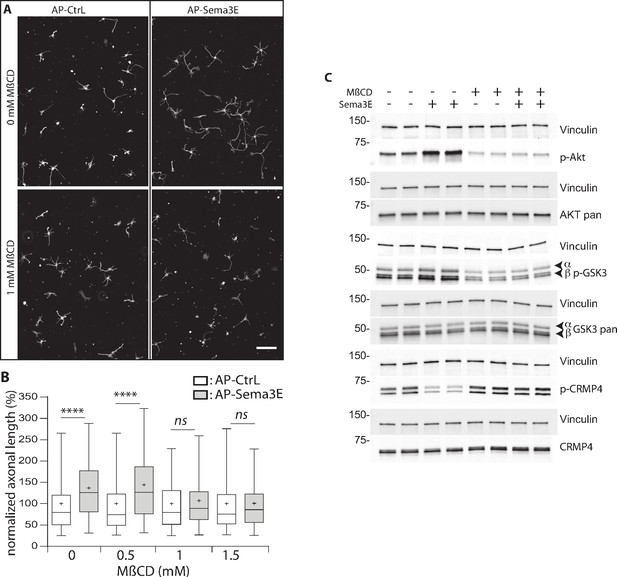
Methyl-beta-cyclodextrin (MβCD) inhibits the downstream Semaphorin-3E (Sema3E) signaling pathway.
(A) Representative images of wild-type (WT) subicular neurons cultured in the absence (AP-CtrL) or presence of 5 nM Sema3E (AP-Sema3E) for 48 hr, with or without MβCD pretreatment (1.5 mM). Cells were fixed and stained with an anti-tubulin antibody to visualize neuronal extensions. Scale bar 100 µm. (B) Quantification of relative axonal lengths in WT subicular neurons cultured in the absence (AP-CtrL) or presence of 5 nM recombinant Sema3E (AP-Sema3E) for 48 hr, following a 2 hr pretreatment with different MβCD concentrations (from 0 to 1.5 mM). Crosses represent the mean, black bars represent the median, boxes represent the 25th and 75th percentiles, and the whiskers represent the 5th and 95th percentiles of values (300 neurons for each condition from two independent cultures). Values were normalized to 100% for control conditions (AP-CtrL). Kruskal-Wallis non-parametric test followed by Dunn’s multiple comparisons, ****p < 0.0001 and n.s. p > 0.05. (C) Representative western blots of subicular neurons exposed or not to 4 mM MβCD for 30 min, followed or not with 5 nM recombinant Sema3E for 11 min. Proteins and phosphorylated proteins were assessed using their corresponding antibodies. n = 2 independent experiments, samples were loaded in duplicate.
-
Figure 9—source data 1
Extended view of panel B and raw data of C.
- https://cdn.elifesciences.org/articles/70361/elife-70361-fig9-data1-v5.zip
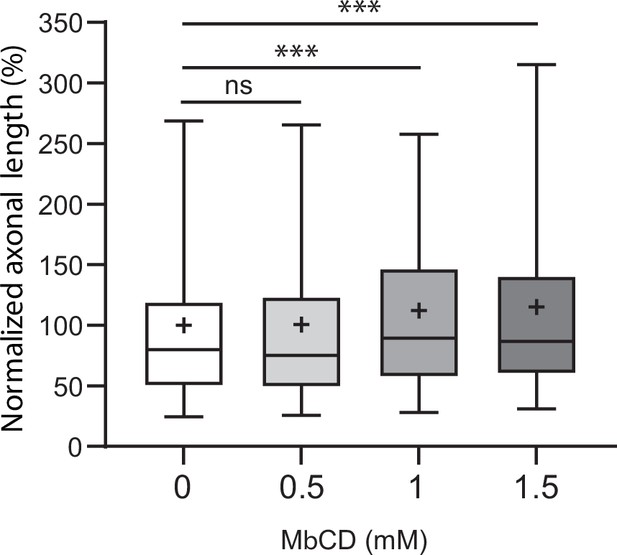
Effect of methyl-beta-cyclodextrin (MβCD) on axonal growth of subicular neurons.
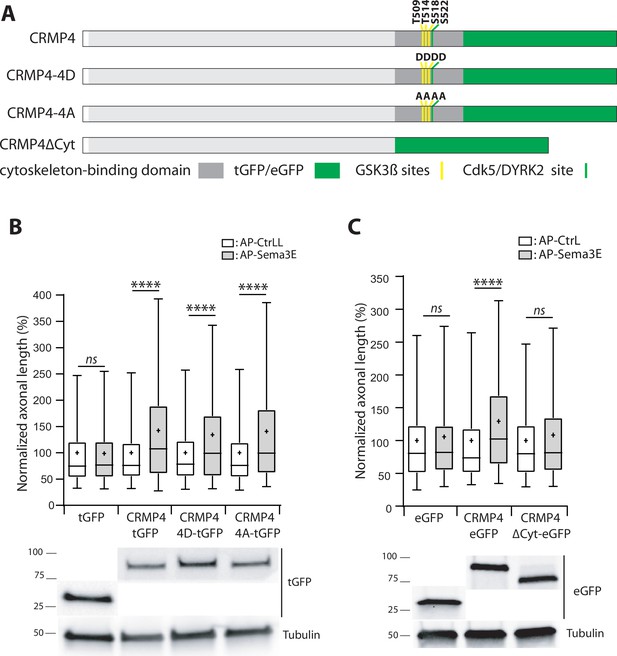
The cytoskeleton-binding domain of collapsin response mediator protein 4 (CRMP4) is required for Semaphorin-3E (Sema3E) growth-promoting activity.
(A) Schematic representations of tGFP- or eGFP-tagged CRMP4 proteins used for rescue experiments in subicular neurons. Cytoskeleton-binding domain (grey rectangle) and the positions of the GSK3β (yellow stripes) and Cdk5/DYRK2 (green stripes) phosphorylation sites are indicated. (B) Quantification of the relative axonal length in CRMP4-KO subicular neurons electroporated with plasmids encoding tGFP, CRMP4-tGFP, CRMP4-tGFP non-phosphorylatable mutant (CRMP4-4A-tGFP), or CRMP4-tGFP phospho-mimetic mutant (CRMP4-4D-tGFP), in the absence (AP-CtrL) or presence of 5 nM Sema3E (AP-Sema3E). Expression levels of each CRMP protein (lower panels). (C) Axonal lengths measured for cultured CRMP4-knockout (KO) subicular neurons electroporated with plasmids encoding eGFP, CRMP4-eGFP, or CRMP4-eGFP mutant lacking the cytoskeleton-binding domain (CRMP4ΔCyt-eGFP), in the absence (AP-CtrL) or presence of 5 nM Sema3E (AP-Sema3E). Expression levels for each CRMP protein (lower panels). In B and C, crosses represent the mean, black bars represent the median, boxes represent the 25th and 75th percentiles, and whiskers represent the 5th and 95th percentiles of values from two independent cultures (B) or three independent cultures (C). Neurons (n = 300 per condition) were randomly selected; for one of the three KO-EGFP CtrL cultures, only 277 neurons could be selected. Values were normalized to 100% for values obtained in control conditions (AP-CtrL). Kruskal-Wallis non-parametric test followed by Dunn’s multiple comparisons, ****p < 0.0001 and n.s. p > 0.05.
-
Figure 10—source data 1
Extended view of panels B-C and raw data of panels B-C.
- https://cdn.elifesciences.org/articles/70361/elife-70361-fig10-data1-v5.zip
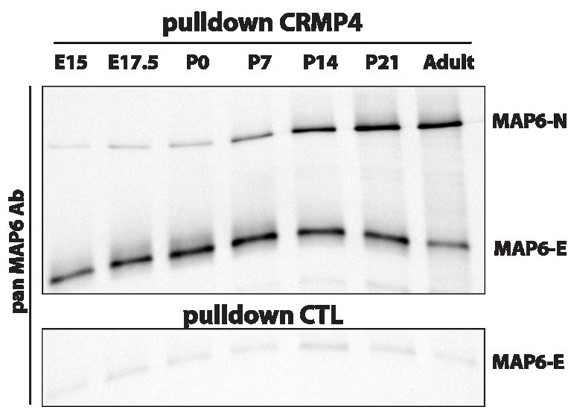
Pulldowns of MAP6-E and MAP6-N from embryonic, post-natal and adult brains using CRMP4-Sepharose (upper panel) and control-Sepharose beads (lower panel).
Western blot using a pan-MAP6 antibody revealed both MAP6-N and MAP-E isoforms.
Additional files
-
Transparent reporting form
- https://cdn.elifesciences.org/articles/70361/elife-70361-transrepform1-v5.docx
-
Source data 1
Statistical analysis Figures 2, 4—6, Figures 9 and 10, Figure 5—figure supplement 1, Figure 9—figure supplement 1.
- https://cdn.elifesciences.org/articles/70361/elife-70361-supp1-v5.zip
-
Supplementary file 1
Summury of MAP6 interacting proteins identified by proteomic analysis.
- https://cdn.elifesciences.org/articles/70361/elife-70361-supp2-v5.xlsx