An unexpected noncarpellate epigynous flower from the Jurassic of China
Abstract
The origin of angiosperms has been a long-standing botanical debate. The great diversity of angiosperms in the Early Cretaceous makes the Jurassic a promising period in which to anticipate the origins of the angiosperms. Here, based on observations of 264 specimens of 198 individual flowers preserved on 34 slabs in various states and orientations, from the South Xiangshan Formation (Early Jurassic) of China, we describe a fossil flower, Nanjinganthus dendrostyla gen. et sp. nov.. The large number of specimens and various preservations allow for an evidence-based reconstruction of the flower. From the evidence of the combination of an invaginated receptacle and ovarian roof, we infer that the seeds of Nanjinganthus were completely enclosed. Evidence of an actinomorphic flower with a dendroid style, cup-form receptacle, and angiospermy, is consistent with Nanjinganthus being a bona fide angiosperm from the Jurassic, an inference that we hope will re-invigorate research into angiosperm origins.
https://doi.org/10.7554/eLife.38827.001eLife digest
From oranges to apples, flowering plants produce most of the fruits and vegetables that we can see on display in a supermarket. While we may take little notice of the poppy fields and plum blossoms around us, how flowers came to be has been an intensely debated mystery.
The current understanding, which is mainly based on previously available fossils, is that flowers appeared about 125 million years ago in the Cretaceous, an era during which many insects such as bees also emerged. But not everybody agrees that this is the case. Genetic analyses, for example, suggest that flowering plants are much more ancient. Another intriguing element is that flowers seemed to have arisen during the Cretaceous ‘out of nowhere’.
Fossils are essential to help settle the debate but it takes diligence and luck to find something as fragile as a flower preserved in rocks for millions of years. In addition, digging out what could look like a bloom is not enough. It is only if the ovules (the cells that will become seeds when fertilized) of the plant are completely enclosed inside the ovary before pollination that researchers can definitely say that they have found a ‘true’ flower.
Now, Fu et al. describe over 200 specimens of a new fossil flower that presents this characteristic, as well as other distinctive features such as petals and sepals – the leaf-like parts that protect a flower bud. Called Nanjinganthus, the plant dates back to more than 174 million years ago, making it the oldest known record of a ‘true’ flower by almost 50 million years. Contrary to mainstream belief, this would place the apparition of flowering plants to the Early Jurassic, the period that saw dinosaurs dominating the planet. This discovery may reshape our current understanding of the evolution of flowers.
https://doi.org/10.7554/eLife.38827.002Introduction
Despite the importance of, the great interest in and intensive effort spent on investigating angiosperms, a controversy remains as to when and how this group came into existence. Since the time of Darwin, some scholars have proposed that angiosperms existed before the Cretaceous (Smith et al., 2010; Clarke et al., 2011; Zeng et al., 2014; Buggs, 2017), although the conclusion ‘there are no reliable records of angiosperms from pre-Cretaceous rocks’ made almost 60 years (Scott et al., 1960) seemed to be recently re-confirmed (Herendeen et al., 2017). Such uncertainty makes answers to many questions about the phylogeny and systematics of angiosperms tentative. Some reports of early angiosperms (i.e., Monetianthus (Friis et al., 2001)) are based on a single specimen, which restricts further testing and confirming. Better and more specimens of early age and with features unique to angiosperms are highly sought-after to test related evolutionary hypotheses. Here, we report an unusual actinomorphic flower, Nanjinganthus gen. nov., from the Lower Jurassic based on the observations of 264 specimens of 198 individual flowers on 34 slabs preserved in various orientations and states (Supplementary file 1). The abundance of specimens allowed us to dissect some of them, thus demonstrate and recognize a cup-form receptacle, ovarian roof, and enclosed ovules/seeds in Nanjinganthus. These features are consistent with the inference that Nanjinganthus is an angiosperm. The origin of angiosperms has long been an academic ‘headache’ for many botanists, and we think that Nanjinganthus will shed a new light on this subject.
Results
Genus
Nanjinganthus gen. nov.
Generic diagnosis
Flowers subtended by bracts. Bracts fused basally. Flowers pedicellate, actinomorphic, epigynous, with inferior ovary. Sepals 4–5, rounded in shape, each with usually 4–6 longitudinal ribs in the center and two lateral rib-free laminar areas, attached to the receptacle rim with their whole bases, surrounding the petals when immature, with epidermal cells with straight cell walls. Petals 4–5, cuneate, concave, each with usually 5–6 longitudinal ribs in the center and two lateral rib-free laminar areas, with rounded tips, surrounding the gynoecium when immature, with epidermal cells with straight cell walls. Gynoecium in the center, unilocular, fully closed by a cup-form receptacle from the bottom as well as sides and by an integral ovarian roof from the above. Style centrally attached on the top of the ovarian roof, dendroid-formed. One to three seeds inside the ovary, elongated oval, hanged on the ovarian wall by a thin funiculus, with the micropyle-like depression almost opposite the chalaza.
Type species
Nanjinganthus dendrostyla gen. et sp. nov.
Etymology
Nanjing- for Nanjing, the city where the specimens were discovered, and -anthos for ‘flower’ in Latin.
Type locality
Wugui Hill, Sheshan Town, Qixia District, Nanjing, China (N32˚08″ 19′ , E118˚58″ 20′) (Figure 1—figure supplement 1).
Horizon
The South Xiangshan Formation, the Lower Jurassic.
Species
Nanjinganthus dendrostyla gen. et sp. nov.
Specific diagnosis
the same as the genus.
Description
The flowers are frequently concentrated and preserved in groups on certain bedding surfaces (Figures 1a–g and 2a–b), although many of them are preserved as isolated individuals on other slabs.
Flower bud
A flower bud is preserved as a coalified compression, 6.4 mm long and 3 mm wide, with characteristic longitudinal ribs on the sepals and petals (Figure 2g). The sepals are estimated to be 1.3–2.2 mm long and approximately 1.8 mm wide (Figure 2g). The petals (including the eclipsed portion) are estimated to be approximately 3.7 mm long (Figure 2g). The receptacle/ovary is approximately 3 mm in diameter (Figure 2g).
Mature flower
The flowers are preserved in various states (including coalification), with cup-form receptacle, epigynous with an inferior ovary, 8.4–10.7 mm in length and 6.8–12.8 mm in diameter, actinomorphic in the bottom and top views (Figures 1a-g, 2a-f,h, 3a-b,d-f, 4a-b,d,g, 5e-i, 6a,f,j,l and 7a,e). The pedicel is approximately 0.76 mm in diameter (Figure 6a,b). Basally fused bracts 0.7–3.7 mm long are observed at the bottom in a few flowers, and a stoma is seen on a bract (Figures 4g-h, 7e,h, and 8h). The receptacle is cup-form, 3–4.8 mm in diameter and 2–4.5 mm high, surrounded by a 0.3 mm thick wall in the bottom and sides, and covered by an ovarian roof from the above (Figures 2h–i, 4d, 5h, 6a–b and 7a,e–f,i). Scales are attached on the sides of the receptacle/ovary (Figures 3a–b, 4a,g–h, 5i and 7a,e,i). The sepals are 1.7–3 mm long and 2.7–4.3 mm wide, with two lateral rib-free laminar areas and usually four longitudinal ribs in the center, and attached to the receptacle rim with their whole bases (Figures 2c-f, 3d-f, 4a,b,d-e, 5i,l, 6l, 7e,i, 9a). The elongated epidermal cells are, 44–156 μm x 33–54 μm, with straight cell walls in the middle region, while isodiametric epidermal cells 16–71 μm x 10–54 μm are seen in the lateral laminar areas of the sepals (Figures 8g, 9h-k and 10d-f). The petals are 3.1–6.6 mm long, 1.9–5.4 mm wide, compressed to only about 11 μm thick, with two lateral rib-free laminar areas, a cuneate base, and 5–6 longitudinal ribs in the center, located inside the sepals on the rim of the receptacle (Figures 2c–f,h,j,4a,b,d,6a,f,7a,e,i,8a–f,9a–g). The ribs are approximately 0.12 mm wide, forking only basally, with elongated epidermal cells with straight cell walls, 32–144 μm x 17–30 μm on the abaxial and 19–72 μm x 13–29 μm on the adaxial (Figures 8a-f, 10a-b). The lateral laminar areas are free of ribs, and each is approximately 1.2 mm wide, with isodiametric epidermal cells 23–64 μm x 18–37 μm (Figures 8e, 9g). A possibly immature stoma is seen on one of the petals (Figure 10c). An unknown organ (staminode?) is seen once on the rim of the receptacle (Figure 6a,m). The ovarian roof is horizontal, with smooth integral outer and inner surfaces, 0.14–0.22 mm thick, with a style vertically located on its center (Figures 4c, 5h, 6f and 7a–c,e–g). The style is 0.3–0.8 mm in diameter, with lateral branches that make the width of the style 3–6 mm (Figures 2h–i, 3a–b, 5i–j, 6a,c and 7a–d). The basalmost pair of the lateral branches appear oppositely arranged along the style (Figures 2h and 3b) while the upper ones appear irregularly arranged (Figures 2i, 6a and 7c–d). There are longitudinal faint striations on the surface of the style (Figures 3c and 5j). There are 1.6–3.6 mm long and 1.7–2.2 mm wide round-triangular scales on the sides of the ovary (Figures 2g, 3b, 4a,g–h and 5i). Each ovary contains one to three seeds that are 0.65–3 mm x 0.5–1.7 mm, elongated or oval-shaped (Figures 2f, 5a,c and 6d,f–l), hanged on the inner wall of the ovary by a 0.08–0.27 mm wide funiculus (Figures 5e and 6d–e). A micropyle-like depression 0.15 × 0.36 mm is seen on a seed (Figure 5a,d).

Siltstone slabs bearing Nanjinganthus.
All bars are 1 cm long. (A) Six flowers (1-6) on the same slab, and an associated triangular leaflet with parallel venation. PB22227. (B) Several flowers on the same slab. 1–3 are shown in detail in Figures 2f and 6d,e. PB22226. (C) Several flowers (1-8) on the same slab and the associated Nilssonia parabrevis (top). PB22220. (D) Several flowers (1-6) on the same slab. 1–3 are shown in detail in Figures 2h and 3a–c. PB22224. (E) Many flowers on the same slab. Some of the numbered ones are shown in detail in later figures. PB22222a. (F) A slab with numerous flowers. PB22221. (G) A slab almost fully covered with flowers. PB22228.

Flowers of Nanjinganthus preserved in different states and their details.
Bar = 1 mm except otherwise annotated. (A) Numerous flowers preserved on a single slab. Some of the numbered ones are detailed in later figures. PB22222B. Bar = 1 cm. (B) Numerous coalified flowers on the same slab. Some of the numbered ones are detailed in Figure 3d–e. PB22223. Bar = 1 cm. (C) Bottom view of Flower 1 in Figure 2a, showing five sepals (s) and five petals (p) with longitudinal ribs. PB22222B. (D) Bottom view of Flower 2 in Figure 2a, showing four sepals (s) and four petals (p) with longitudinal ribs. PB22222B. (E) Bottom view of the flower in Figure 3f, showing a sepal (s) and three petals (p) radiating from the center, which is obliquely broken to show the relationship among the sepals and petals as in Figure 2j. PB22278. (F) Top view of Flower 1 in Figure 1b with sepals (s), petals (p), and seeds (arrow, enlarged in Figure 6h) inside the receptacle. PB22226. (G) Side view of a flower bud (Flower 1 in Figure 2b) with longitudinal ribs (arrows) on the sepals (s) and petals (p). PB22223. (H) Side view of Flower 1 in Figure 1d, showing a receptacle (h), perianth (black arrows), and a dendroid style (white arrow). PB22224. (I) Side view of Flower 15 in Figure 1e, without sepals or petals. PB22222a. Bar = 1 mm. (J) Detailed view of the flower shown in Figure 2e, showing the arrangement of three petal bases (1-3) inside the sepals (s). These petals bases correspond to the three petals (1-3) in Figure 2e. PB22278.
Individuals of Nanjinganthus.
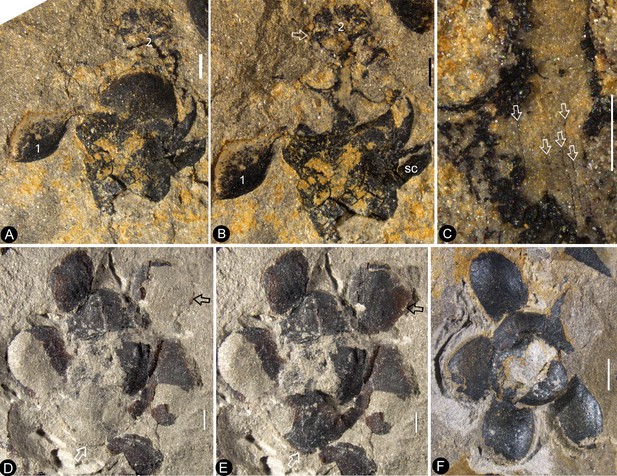
Bar = 1 mm except otherwise annotated. (A–C, PB22224) (A) Flower 2 in Figure 1d (before the dégagement), showing the petal (1) and style (2) still embedded in the sediments. (B The same flower as in Figure 3a, after dégagement, showing the exposed dendroid style (white arrow) and petal (1), and the scale (sc) on the side of receptacle. (C) Detailed view of the style shown in Figure 3b with faint striations (arrows). Bar = 0.5 mm. (D–E) Flower 2 in Figure 2b after and before the organic material of the sepals (white arrows) and petals (black arrows) were removed for cuticle analysis. PB22223. (F) Bottom view of a flower before processing. Internal details are shown in Figure 2e,j. PB22278.

Nanjinganthus flowers preserved in various orientations and states.
Bar = 1 mm except otherwise annotated. (A) An oblique longitudinally split flower (Flower 11 in Figure 1e) with scales (sc), sepals (s), and petals (p). PB22222a. (B) A longitudinally split flower (Flower 12 in Figure 1e) with sepals (s) and petals (p). PB22222a. (C) Integral surface of an ovarian roof with a scar (arrow) left by a broken off style, from the flower shown in Figure 5h. PB22279. Bar = 0.5 mm. (D) Bottom view of a flower (Flower 14 in Figure 1e) with three sepals (s) and five petals (p) visible. PB22222a. (E) One of the sepals in Figure 4d, showing longitudinal ribs forking (arrow). PB22222a. (F) One of the petals in Figure 4d, showing longitudinal ribs. PB22222a. (G) Side view of a flower, showing scales (sc) on the ovary side and connate bracts (b) at the bottom. PB22229. (H) Detailed view of the connate bracts (b) and scales (sc) in Figure 4g. Note the outline (white line) of the fused bracts. PB22229. (I) The locule surrounded by the ovary wall (arrows) of the flower shown in Figure 4d. PB22222a.
In situ seeds and flowers.
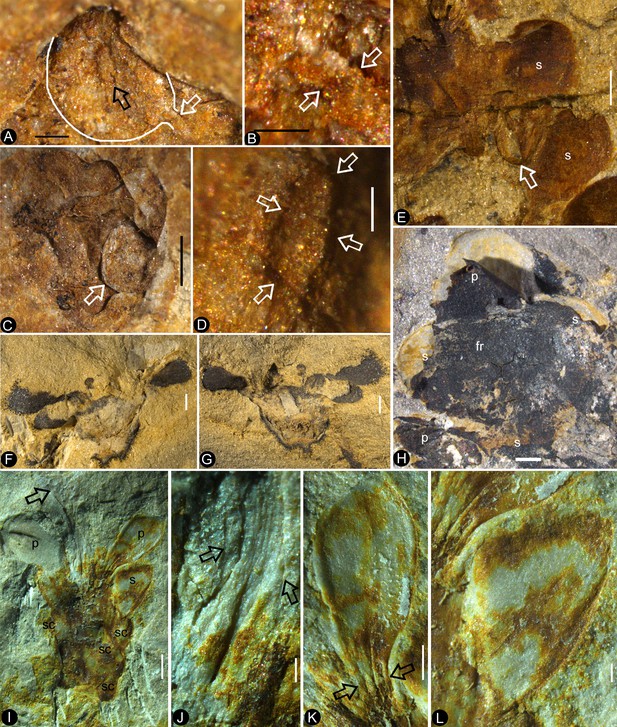
Bar = 1 mm except otherwise annotated. (A) A seed (outlined) inside the ovary of Flower 16 in Figure 1e. Note the oboval micropyle (black arrow) and funiculus (white arrow). PB22222a. Bar = 0.2 mm. (B) Detailed view of the funiculus (between the arrows) of the seed in Figure 5a. PB22222a. Bar = 0.1 mm. (C) A seed (detailed in Figure 6i) inside the ovary of Flower 7 in Figure 1e. PB22222a. (D) Detailed view of the oval micropyle (arrows) of the seed in Figure 5a. PB22222a. Bar = 0.1 mm. (E) A seed (arrow, detailed in Figure 6d–e) inside the receptacle in Flower two in Figure 1b. PB22226. (F, G) Two facing parts of the same flower (Flower 10 in Figure 1e). PB22222a. (H) Top view of a flower with organically-preserved sepals (s), petals (p) and integral ovarian roof (fr), which is detailed in Figure 4c. PB22279. (I) Side view of a longitudinally split flower with scales (sc) on ovary side, sepals (s), petals (p) and partially preserved style (arrow). PB22489. (J) Detailed view of basal portion of the style (between arrows) arrowed in Figure 5i, with faint longitudinal striations. PB22489. Bar = 0.2 mm. (K) Detailed view of the narrowing base (between arrows) of the right petal in Figure 5i. PB22489. Bar = 0.5 mm.( L) Detailed view of a sepal in Figure 5i. PB22489. Bar = 0.2 mm.

Dendroid style, in situ seeds, and details of flowers.
PB22222a, Bar = 1 mm except otherwise annotated. (A) A longitudinally split flower (counterpart of Flower 10 in Figure 1e, the same as in Figure 5f–g) showing the sepal (s) and petals (p), style base (white arrow), and an unknown organ (black arrow). (B) Detailed view showing the pedicel (lower arrow) terminating at the bottom of the ovary in Figure 6a. Note the level of ovarian roof (upper arrow). Bar = 0.5 mm. (C) Detailed view of the basal portion of the style marked by white arrow in Figure 6a. Bar = 0.5 mm. (D) A seed (white line) hanging by its funiculus (between arrows) on the ovarian wall of the Flower 2 in Figure 1b. PB22226. Bar = 0.5 mm. (E) Detailed view of the funiculus (between arrows) of the seed in Figure 6d. PB22226. Bar = 0.1 mm.( F) Top view of Flower 8 in Figure 1e with sepals and petals surrounding the ovary containing two seeds (s). Note the residue (arrows) of the ovarian roof. (G) Detailed view of one of the oval seeds (s) inside the ovary in Figure 6f. Bar = 0.2 mm. (H) Two seeds (white line), one overlapping the other, inside the ovary shown in Figure 2f. PB22226. Bar = 0.2 mm. (I) An oval seed (white line) inside the ovary of Flower 7 in Figure 1e. Bar = 0.2 mm. (J) Detailed view of Flower 1 in Figure 1g, showing seeds within ovary. PB22228. (K) Detailed view of three seeds (1-3) inside the ovary of the flower shown in Figure 6j. PB22228. Bar = 0.5 mm. (L) Top view of a flower showing petals (p), sepal (sp), seed (s) visible under the ovarian roof (fr). PB22222d. (M) Detailed view of the unknown organ (staminode?) marked by the black arrow in Figure 6a. Bar = 0.5 mm.

The flowers and their internal details.
(A-C) (E-I) stereomicroscopy; (D), micro-CL. Bar = 1 mm except otherwise annotated. (A) A flower carefully dégaged to expose the details of the gynoecium. Note the petals (p) and a style (arrow) in the center. PB22282. (B) Detailed view of the style in Figure 7a, showing its connection (arrows) to the ovarian roof (fr). PB22282. Bar = 0.5 mm. (C) Distal portion of the same style as in Figure 7b, showing its connection with the ovarian roof (fr) and dendroid form with lateral branches (arrows). PB22282. Bar = 0.5 mm. (D) Micro-CL slice 1169 showing a perianth element (black arrow) and branches (white arrows) of the style, embedded in sediments and thus invisible to naked eyes, of Flower 4 in Figure 1e. PB22222a. (E–I) PB22281. (E) Side view of an organically-preserved flower with sepals (s) and petals (p). Note the dark organic material in the ovary (o) and some sepals. The foreground portion of the receptacle has been removed (compare with Figure 7i), to show the details in Figure 7f–h. (F) Detailed view of the receptacle/ovary in Figure 7e. Note the ovarian roof (fr) preventing the outside (above) sediment (yellow color) from entering the ovarian locule. Bar = 0.2 mm. (G) Detailed view of the solid organically-preserved ovarian roof (fr) with integral outer (upper arrow) and inner (lower arrow) surfaces. Bar = 0.1 mm. (H) Bottom portion of the flower in Figure 7i, showing subtending bracts (br, arrows). Bar = 0.5 mm. (I) The flower in Figure 7e, before removing the foreground portion of the ovary.
Details of the sepal and petal.
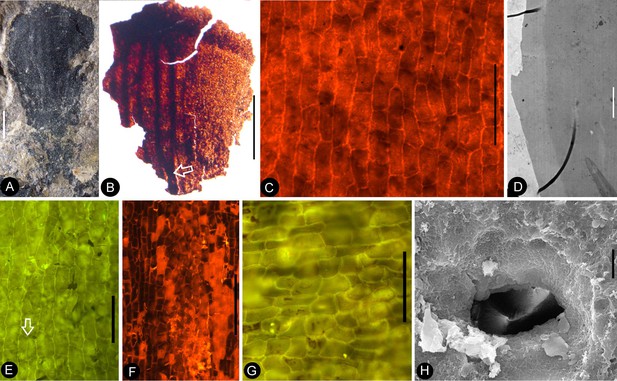
(A-B) stereomicroscopy; (C) (E-G) fluorescence light microscopy; (D) TEM; (H) SEM. Bar = 1 mm except otherwise annotated. (A) A petal with a narrowing base. PB22280. (B) A partial petal from the Flower in Figure 3d–e, with the longitudinal rib (to the left) forking at the base (arrow) and the rib-free laminar area to the right. PB22223. (C) Elongated epidermal cells of the petal in Figure 8b . PB22223. Bar = 0.1 mm. (D) Transmission electron microscope view showing the cuticle (left, light color) of a petal. PB22223. Bar = 2 μm. (E) Elongated epidermal cells not in strict longitudinal files in the laminar portion of the petal in Figure 8b . Note the two newly formed epidermal cells (arrow). PB22223. Bar = 0.1 mm. (F) Ribs with elongated epidermal cells (left and right) alternating the between region with less elongated cells (middle) of the petal in Figure 8b . PB22223. Bar = 0.2 mm. (G Elongated (above) and isodiametric (below) epidermal cells on the sepal of Flower in Figure 3d–e. PB22223. Bar = 0.1 mm. (H) A stoma on the bract of the flower (marked by white arrow in Figure 2h). PB22224. Bar = 5 μm.
Petal and details of Nanjinganthus.
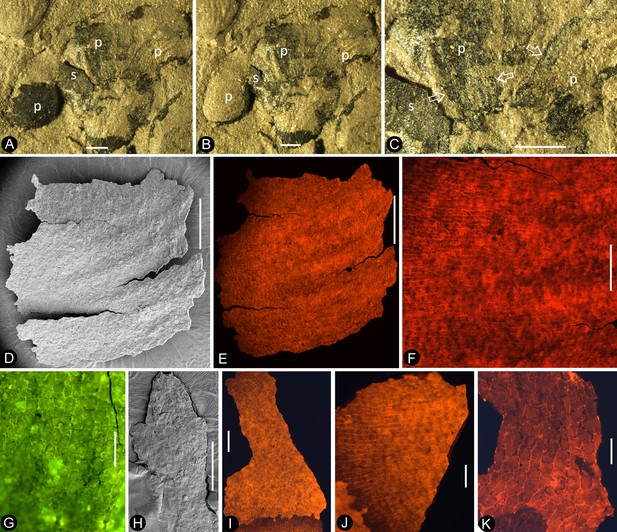
(A-C) light stereomicroscopy; (D) (H) SEM; (E-G) (I-K) fluorescence light microscopy. PB22223. Bar = 1 mm except otherwise annotated. (A) Side view of Flower 3 in Figure 2b, showing the arrangement of the petals (p) and sepal (s). (B) The same flower as in Figure 9a. Note that some organic material of the petal has been removed for detailed observation. (C). Margins (arrows) of the petal (p) with cuneate base and their relationship to the sepal (s). (D) The petal removed from Figure 9a. SEM. Bar = 0.5 mm. (E) Cellular details of the petal in Figure 9d Bar = 0.5 mm. (F) Elongated epidermal cells arranged in files, enlarged from Figure 9e. Bar = 0.2 mm. (G) Isodiametric epidermal cells in the laminar area portion of the petal in Figure 9e. Bar = 0.1 mm. (H) A fragment of the sepal seen in Figure 9a. Bar = 0.5 mm. (I) Cellular details of the sepal in Figure 9h. Bar = 0.2 mm. (J) Elongated epidermal cells arranged in files on the sepal in Figure 9i. Bar = 0.1 mm. (K) Isodiametric epidermal cells on the laminar area of the sepal in Figure 9a. Bar = 0.1 mm.
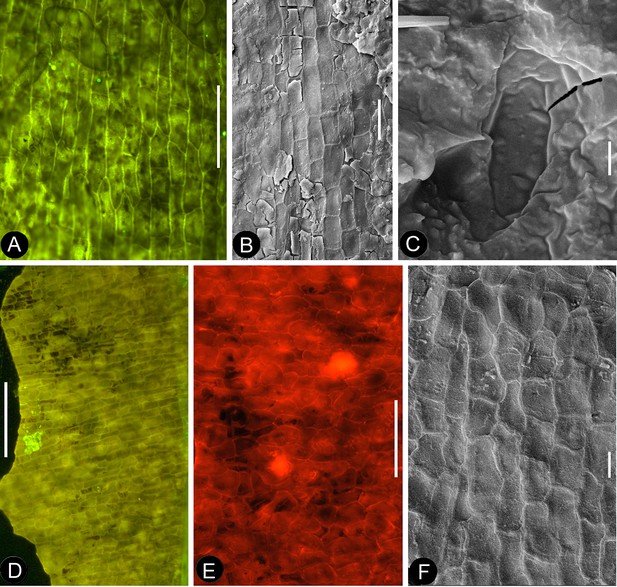
Cuticular details of Nanjinganthus.
A, D-E, Fluorescence light microscopy; B-C, F, SEM. PB22223. (A) Elongated epidermal cells in longitudinal files in the middle portion of the petal in Figure 8b. Bar = 0.1 mm. (B) Elongated epidermal cells on the rib of the petal in Figure 8b. Bar = 50 μm. (C) A possible stoma on the petal shown in Figure 8b. Bar = 2 μm. (D) Elongated epidermal cells in files on the sepal of flower in Figure 3d–e. Bar = 0.2 mm. (E) Isodiametric epidermal cells on the sepal of flower in Figure 3d–e. Bar = 0.1 mm. (F) Isodiametric epidermal cells on the sepal of flower in Figure 3d–e. Bar = 20 μm.
Holotype
Figure 2d (PB22222B).
Isotypes
Figure 6a,f (PB22222a), Figure 7e–i (PB22281), Figure 5h (PB22279).
Specimens
PB22222-PB22229, PB22236, PB22238, PB22241-PB22243, PB22245-PB22247, PB22256-PB22260, PB22278-PB22282, PB22489.
Etymology
dendrostyla, for ‘tree-like’ (dendri-) and ‘style’ (-stylus) in Latin.
Remarks
The receptacle is ‘the axis of a flower on which the perianth, androecium and gynoecium are borne’ (Stevens, 2018). This is the definition followed here. The important characteristic of the receptacle in Nanjinganthus is its cup form, a form frequently seen in more derived angiosperms according to the APG system.
A dendroid style is seen in ten flowers (four in PB22224, Figures 2h and 3a–b; four in PB2222a, Figures 2i, 5f–g, 6a and 7d; one in PB22282, Figure 7a–c; one in PB22489, Figure 5i–j). The repeated occurrences of such an unexpected feature in the specimens of Nanjinganthus underscore its truthful existence. The dendroid-form distal portion of the gynoecium may be branched stigmas in Nanjinganthus. But it is possible that these lateral appendages on the style are actually pollen sac complexes, as are similarly attached on the style in extant Malvaceae (Judd et al., 1999). We have performed a meticulous fluorescence microscopic examination of this structure and found no trace of pollen grains, reducing the possibility that these lateral branches are clusters of pollen sacs, which is the case seen in some angiosperms (Malvaceae). A branched distal projection is apparently lacking in all known gymnosperms, but it has been seen some derived angiosperms, such as Passifloraceae, Poaceae and Euphorbiaceae (Heywood, 1978). One of the advantages of a branched style is the increased receptive area, which is conducive to anemophilous pollination. The occurrence of such feature in Nanjinganthus might suggest that Nanjinganthus had yet not established a close cooperation with animals (insects). However, it is noteworthy that this feature is not seen among extant basal angiosperms sensu APG (Chase et al., 2016). Considering the extremely early age of Nanjinganthus, we refrain from correlating Nanjinganthus with assumed derived taxa (Malvaceae and Rosaceae). We hope the future research may shed more light the nature of this part of Nanjinganthus.
We have not seen any trace of the carpels typical of Magnoliales, which were previously believed by some to represent ancestral angiosperms. The seeds are physically enclosed by the cup-form receptacle and ovarian roof in Nanjinganthus. This constitutes the foundation based on which we justify our interpretation of Nanjinganthus as an angiosperm. The lack of carpel typical of Magnoliales cannot prevent Nanjinganthus from being an angiosperm as many angiosperms are actually ‘acarpellate’ (Heads, 1984; Sattler and Lacroix, 1988). It is noteworthy that, at least in some of basal angiosperms such as Nymphaea (Nymphaeales) (Taylor, 1991; Taylor, 1996) and derived angiosperms such as Cactaceae (Boke, 1964), the ovary is inferior and the seeds are attached to the ovarian walls. Whether the ovaries in these taxa share similar derivation pathway is a question worthy of further investigation.
Four terms are used to describe the foliar parts in Nanjinganthus, namely, bract, scale, sepal, and petal. These terms are used according to the following demarcations and definitions. Bracts designate the foliar parts subtending the ovary. The scales are the foliar parts attached to the sides of the ovary. The sepals are those foliar parts attached to the rim of the receptacle with their whole bases. And the petals are foliar parts with narrowing bases attached to the receptacle rim and inside the sepals. Similar occurrence of bracts, sepals and petals is seen in some extant flowers (Figure 5—figure supplement 1).
The enclosure of the seeds is fulfilled by the cup-form receptacle from the bottom and the structure here-called ‘ovarian roof’ (preserved complete in Figures 4c, 5h and 7e–g, but partially preserved in Figures 2f, 5c and 6f,j,l) from the above. The intact ovarian roof is clearly seen in the side view (Figure 7f–g) and in surface view (Figures 4c and 5h), in the latter case the seeds inside ovary are fully eclipsed by the ovarian roof. The ovarian roof is partially lost in Figure 6l, in which a central portion of the ovarian roof broke off revealing one of the seeds inside the ovary. The ovarian roof is almost completely lost (but still with some of its residue) in Figure 6f, and finally fully lost in Figures 2f and 6j–k, in which the seeds are plainly visible. This series of varying preservation status of ovarian roof suggests that the ovarian roof has fully enclosed the ovules in its original status, and the loss of ovarian roof and exposure of seeds are artifacts due to preservation.
We cannot recognize the maturity of the ovules/seeds in Nanjinganthus, the length about 1 mm suggests that they are most likely to be seeds rather than ovules, therefore we prefer to use the term ‘seed’ rather ‘ovule’ throughout this paper. The number of seeds in Nanjinganthus is variable. According to our observation, it may be one (not shown), two (Figure 6f–i), or even three (Figure 6j–k).
Discussion
Alternative interpretations
The Mesozoic was an age of gymnosperms, so the Jurassic age of Nanjinganthus makes it necessary to compare Nanjinganthus with common fossil gymnosperms frequently seen in the Mesozoic first. The potential candidates for Nanjinganthus include Caytoniales, Corystospermales, Ginkgoales, Czekanowskiales, Coniferales, Iraniales, Pentoxyales, Bennettitales, and Gnetales.
Caytonia is a very intriguing fossil plant that has been frequently compared with angiosperms (Thomas, 1925; Doyle, 2006). Regardless of its ultimate gymnospermous affinity (Thomas, 1925; Harris, 1933; Harris, 1940; Nixon et al., 1994), Caytonia can be easily distinguished from Nanjinganthus by its cupule with an adaxial basal opening, bilateral reproductive organs, and lack of both a dendroid style and foliar appendages in its reproductive organs.
Corystospermales is usually considered as a Mesozoic seed fern group, unlike Caytonia, the cupules in most Corystospermales open on the abaxial base and are rarely compared with angiosperms (Taylor et al., 2009). Corystospermales can be easily distinguished from Nanjinganthus by their cupule which has an abaxial basal opening, bilateral reproductive organs, and the lack of both a dendroid style and foliar appendages in the reproductive organs.
Ginkgoales diversified greatly during the Mesozoic, and unlike extant Ginkgo, the Mesozoic relatives of Ginkgo are well represented by their reproductive organs, which are composed of seeds in clusters (Zhou and Zheng, 2003). Ginkgoales can be easily distinguished from Nanjinganthus by their clustered naked seeds, and lack of a dendroid style in the reproductive organs.
Czekanowskiales are a unique group of fossil plants restricted to the Mesozoic. Their reproductive organs are bivalvate cupules containing two rows of seeds. Czekanowskiales can be easily distinguished from Nanjinganthus by their bivalvate cupules, bilateral reproductive organs, and lack of both a dendroid style and foliar appendages in the reproductive organs.
Irania is the single genus of the Iraniales, which is assumed to have borne clusters of pollen sacs and fruits, from the Triassic-Jurassic (Schweitzer, 1977). Although no seeds have been observed in Irania, it is suspected to be an angiosperm. Its female and male parts are not concentrated on the same axis, and do not constitute a flower-like structure, and it is unknown whether the seeds are enclosed. These features distinguish Irania from Nanjinganthus.
Pentoxylales are Mesozoic woody fossil plants characterized by a stem with five steles (Taylor et al., 2009). Their reproductive organs are cones with numerous naked orthotropous seeds helically arranged around the axes of their cones. Pentoxylales can be easily distinguished from Nanjinganthus by their cones which are devoid of any foliar appendages and the lack of a dendroid style.
Bennettitales are important Mesozoic gymnosperms that are frequently related to angiosperms (Crane, 1985; Rothwell et al., 2009). Their reproductive organs are characterized by orthotropous ovules with micropylar tubes dispersed among interseminal scales, and these parts are helically arranged along the cone axis. Bennettitales can be easily distinguished from Nanjinganthus by their cones with ovules bearing micropylar tubes, and lack of a dendroid style (Taylor et al., 2009).
Gnetales are important gymnosperms that diversified once in the Mesozoic, among which Gnetum has leaves that are difficult to distinguish from eudicots (Martens, 1971; Biswas and Johri, 1997). A characteristic feature of Gnetales is their decussate arrangement of leaves and cone parts. Like in Bennettitales, the reproductive organs of Gnetales are characterized by orthotropous ovules with micropylar tubes surrounded by scales. Like Bennettitales, Gnetales can be easily distinguished from Nanjinganthus by their cones with ovules with micropylar tubes and lack of a dendroid style.
Besides the above comparison among female organs of seed plants, it is noteworthy that male cones in some conifers demonstrate a certain resemblance to Nanjinganthus, although such a comparison appears spurious when the presence of seeds in Nanjinganthus is taken into consideration. The bud-like Nanjinganthus (Figures 2g, 4a–b,g and 5i) appears similar to male cones of Microbiota decussata (a3 in Figure 2 of Schulz et al., 2014) and Thuja plicata (f2 in Figure 2 of Schulz et al., 2014). Mature Nanjinganthus (Figures 2h–i, 3a–b, 5f–g, 6a and 7a,e,i) appear like the male cone of Sequoia sempervirens, Taxus floridana, and Tsuga canadensis (e2, e5, f4, respectively, in Figure 2 of Schulz et al., 2014). The sporangiophores in these taxa are arranged around the cone axis and appear dendrioid, and the scales at the base appear like the sepals/petals in Nanjinganthus. But the cup-form receptacle with seeds inside plus the lack of pollen grains in the distal dendroid part distinguish Nanjinganthus from all these male cones. As in female cones, these male cones also have cone axes penetrating the cones from the bottom to the tip and thus are different from Nanjinganthus in which the pedicel stops at the bottom of the organ (ovary) (Figure 6b) and the style starts above the ovarian roof (Figure 4c, 5h, 6a-c, 7a-c). In addition, the spatial distribution and furcation of the vascular bundles in the sepals and petals of Nanjinganthus (Figures 2c–d,4e–f,9a–b) are distinct from those seen in bracts and scales in coniferous cones.
From the above comparison, we infer that none of the known gymnosperms, fossil or extant, are comparable to Nanjinganthus. The enclosed seeds distinguish Nanjinganthus from gymnosperms, which are not supposed to enclose their ovules in such a way (Table 1).
Comparison between Nanjinganthus and Mesozoic gymnosperms.
https://doi.org/10.7554/eLife.38827.018| Nanjinganthus | Caytonia | Bennettitales | Corystospermales | Ginkgoales | Coniferales | Iraniales | Czekanowskiales | Pentoxylales | |
|---|---|---|---|---|---|---|---|---|---|
| Symmetry | Radial | Bilateral | Radial | Bilateral | Radial | Radial | Radial? | Bilateral | Radial? |
| With foliar appendages | Yes | No | No | No | No | ? | No | No | No |
| Enclosed seed | Yes | No | No | No | No | No? | ? | No | No |
| Opening in female part | No | Adaxial basal | Terminal? | Adaxial basal | N/A | N/A | ? | Distal slit | ? |
| Penetrating cone axis | No | Yes | Yes | Yes | ? | Yes | ? | Yes | Yes |
There are several reports of Jurassic angiosperms, including Schmeissneria (Wang et al., 2007), Xingxueanthus (Wang and Wang, 2010), Juraherba (Han et al., 2016), and Euanthus (Liu and Wang, 2016). These genera are from the Middle-Late Jurassic of northeastern China. The cup-form receptacle, inferior ovary, and dendroid style distinguish Nanjinganthus from all these Jurassic peers, and justify Nanjinganthus as a new genus of angiosperm.
Although multiple characters have been suggested to identify fossil angiosperms (Herendeen et al., 2017), angio-ovuly before pollination is the key character that distinguishes an angiosperm from other seed plants (Tomlinson and Takaso, 2002; Wang, 2010a; Wang, 2018). This criterion has been repeatedly applied to identify fossil angiosperms (i.e. Archaefructus (Sun et al., 1998), which initially had no other features (stamen, venation, pollen grains) but enclosed seeds to support their angiospermous affinity). The integral ovarian roof of Nanjinganthus has no opening (Figures 4c and 5h). After burial, this ovarian roof can block the sediment from entering the ovarian locule (Figure 7e–g). That this space remained free of sediment suggests a full enclosure of the ovules/seeds, securing an angiospermous affinity for Nanjinganthus.
The radial arrangement of two whorls of foliar parts (sepals and petals) in Nanjinganthus is very similar to those of flowers in extant angiosperms (Figures 1a–b and 2a–f), while the above comparison with known gymnosperms emphasizes that Nanjinganthus cannot be interpreted as a gymnosperm. Furthermore, Nanjinganthus satisfies all thirteen definitions of flowers advanced by various authors (Bateman et al., 2006). These features again are consistent with the angiospermous affinity of Nanjinganthus (Figure 11).
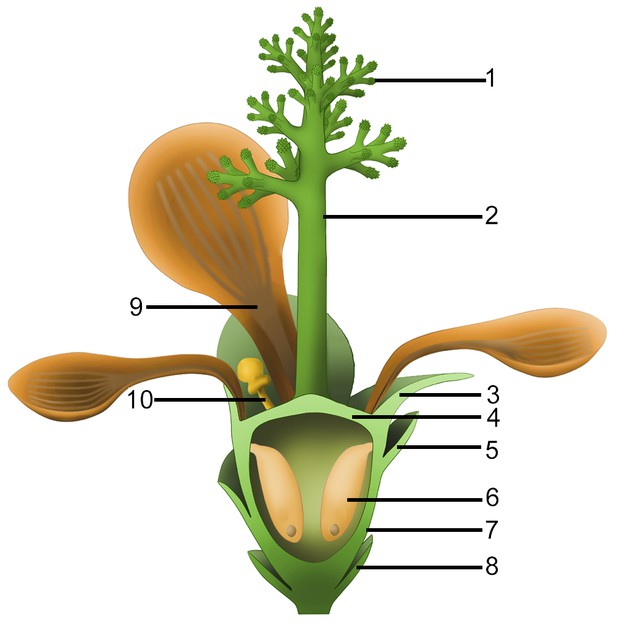
Idealized reconstruction of Nanjinganthus.
1, branches of dendroid style; 2, dendroid style; 3, sepal; 4, ovarian roof; 5, scale; 6, seed; 7, cup-form receptacle/ovary; 8, bract; 9, petal; 10, unknown organ (staminode?).
There have been several suggested models of ancestral angiosperms (Arber and Parkin, 1907; Cronquist, 1988; Endress and Doyle, 2015; Sauquet et al., 2017). These models were drawn more or less after the assumed basalmost living angiosperms, either Magnolia or Amborella. The common features of these model plants include apocarpy, superior ovary, lack of obvious style, etc. However, these features are rarely seen in Nanjinganthus or other early angiosperms (Wang et al., 2007; Wang and Zheng, 2009; Wang, 2010b; Wang, 2018; Han et al., 2013; Han et al., 2016; Han et al., 2017; Liu and Wang, 2016; Liu and Wang, 2017; Liu et al., 2018; Liu and Wang, 2018). Instead, an inferior ovary, a feature unexpected by, at least, most theories of angiosperm evolution, is clearly seen in Nanjinganthus and quite many Early Cretaceous flowers (Friis, 1984; Friis, 1990; Friis et al., 2011). This discrepancy between fossil observation and theories suggests EITHER that inferences based on living plants have limited capability of ‘predicting’ past history, OR that angiosperms originated polyphyletically, each lineage has followed a different evolution route, and Nanjinganthus represents one of the many, OR that angiosperms have a history that dates back to a time much earlier than the Cretaceous, and Nanjinganthus is one of the many derived from such assumed ancestor, OR a combination of these. Whatever the implications are, the currently dominant theories of angiosperm evolution apparently need to be reassessed.
Most Nanjinganthus specimens are concentrated on certain bedding surfaces, and over 50 individual flowers are preserved on a single slab (Figures 1a–g and 2a–b), suggesting that Nanjinganthus may have flourished and dominated a particular niche, although Nanjinganthus played only an inferior role in the broader Jurassic ecosystem. The concentrated preservation of delicate flowers is more likely a result of autochthonous preservation, suggesting a habitat very close to water for Nanjinganthus.
Various studies (including palaeobotany) on the South Xiangshan Formation in the last century (Hsieh, 1928; Li et al., 1935; Sze and Chow, 1962; Zhou and Li, 1980; Cao, 1982; Cao, 1998; Cao, 2000; Wang et al., 1982; Huang, 1983; Huang, 1988; Ju, 1987) and our palynological analysis as well as U/Pb dating (Figure 1—figure supplement 1; Supplementary file 2; Santos et al., 2018) suggest a latest Early Jurassic age for Nanjinganthus. Together with the ‘unexpectedly’ great diversity of angiosperms in the Early Cretaceous (Sun et al., 1998; Sun et al., 2001; Sun et al., 2002; Leng and Friis, 2003; Leng and Friis, 2006; Ji et al., 2004; Wang and Zheng, 2009; Wang and Zheng, 2012; Wang and Han, 2011; Han et al., 2013; Han et al., 2017; Wang, 2015; Liu et al., 2010a), pollen grains indistinguishable from angiosperms in the Triassic (Hochuli and Feist-Burkhardt, 2004; Hochuli and Feist-Burkhardt, 2013), a bisexual flower from the Jurassic (Liu and Wang, 2016), and an herbaceous angiosperm from the Middle Jurassic (Han et al., 2016), the unexpectedly early age of Poaceae (Prasad et al., 2011; Wu et al., 2018) and Solanaceae (Wilf et al., 2017), Nanjinganthus with over 200 specimens is consistent with a pre-Cretaceous origin of angiosperms.
The systematic position of Nanjinganthus is now apparently open to further investigation, although it demonstrates a certain resemblance to Pentapetalae sensu (Judd et al., 2016). We cannot determine whether Nanjinganthus stands for a Jurassic stem group of angiosperms that started their radiation later in the Cretaceous or a lateral branch leading to an evolutionary dead end. It is premature to determine its phylogenetic position before more information of contemporaneous peers is available, although we welcome all phylogeneticists to evaluate Nanjinganthus in their own ways and perspectives.
Conclusion
Nanjinganthus is recognized based on at least 198 individual flower fossils from the Early Jurassic that are preserved in various states and orientations. We infer that the seeds were enclosed by a cup-form receptacle and an ovarian roof, traits which suggest an angiospermous affinity for Nanjinganthus. It would be intriguing to figure out in future research whether Nanjinganthus represents a stem group, a group derived from more ancient ancestors, or an evolutionary dead end of polyphyletic angiosperms. We hope that the discovery of Nanjinganthus will re-invigorate research on the origin and early history of angiosperms.
Materials and methods
Geological background
Request a detailed protocolInitially, what is now known as the Xiangshan Group was called the ‘Nanking Sandstein’ that was put in the Upper Carboniferous by Richthofen (1912). Liu and Chao (1924) thought that the Nanking Sandstein belonged to the Jurassic and renamed it the ‘Tsung Shan Formation’. Hsieh (1928) subdivided the Tsungshan Formation into six units, namely, in ascending order, Huang Ma Ching Shale, Quartzitic Conglomerate, Tzu Hsia Tung Series, Lingkusssu Shale, Light Yellow Sandstone, Variegated Sandstone and Shale. Li et al. (1935) found fossil plants including Equisetites, Neocalamites, Cladophlebis, Otozamites, Pterophyllum, Dictyophyllum, Pagiophyllum, Baiera guilhaumatii, and Podozamites lanceolatus. They also changed the ‘Tsungshan Formation’ to ‘Xiangshan Layers’, and regarded its age as being the Early Jurassic. Sze and Chow (1962) used the term ‘Xiangshan Group’ for the previous ‘Xiangshan Layers’, and this has been the convention followed ever since. The age of the Xiangshan Group has been concluded by various authors to range from the Late Triassic to the Middle Jurassic. Ju (1987) divided the Xiangshan Group into a lower South Xiangshan Formation and an upper North Xiangshan Formation, respectively. The standard section of the lower part of the Xiangshan Group (the South Xiangshan Formation, the Lower Jurassic) is 394 meters thick in South Xiangshan in Nanjing, and has yielded abundant fossil plants. The standard section of the upper part of the Xiangshan Group (the North Xiangshan Formation, the Middle Jurassic) is in North Xiangshan in Nanjing. It is 1005 meters thick, and has only yielded a few stem fossils (Ju, 1987). Based on fossil plants, Cao (1982) thought that the age of the South Xiangshan Formation could not be later than the Early Jurassic, and considering that the early Early Jurassic flora (in the middle and lower parts of the Guanyintan Formation in southwest Hunan) (Zhou and Li, 1980) is biostratigraphically below the South Xiangshan Formation, Cao (1982) regarded the age of the lower part of Xiangshan Group (=South Xiangshan Formation) as the middle-late Early Jurassic.
The South Xiangshan Formation (lower part of the Xiangshan Group) has yielded abundant bivalve and plant fossils. Its outcrops are scattered in Jiangning, Longtan, and Zhenjiang (all in the suburbs of Nanjing). In these areas, the outcrops are well exposed and especially fossiliferous near the South Xiangshan and Cangbomen regions. The formation includes sandstones, siltstones, shales, carbonaceous shales, and coal seams. There are abundant plant fossils in the South Xiangshan Formation, and almost all of the plants in the Xiangshan Group are from this formation. Various authors have collected fossil plants of the Xiangshan Flora (Cao, 1982; Cao, 1998; Cao, 2000; Wang et al., 1982; Huang, 1983; Huang, 1988; Ju, 1987). According to Cao (1982), Cao (1998), Cao (2000), Wang et al. (1982), and Ju (1987), the Xiangshan Group includes at least 46 genera of plants and is very similar to the flora of the Hsiangchi Group in western Hubei. Cycadophytes (34%) dominate the flora, and ferns are the second most dominant group (20%), among which Dipteridaceae play an important role. Ginkgoales are also abundant (19%) (Ju, 1987). The important and frequently observed taxa include Hysterites, Selaginellites, Equisetites cf. lateralis, E. aff. multidentatus Oishi, E. sarrani (Zeiller) Halle, Neocalamites hoerensis (Schimper) Halle, N. dangyangensis Chen, Marattiopsis asiatica Kawasaki, M. hoerensis (Schimper) Schimper, Todites goeppertianus (Münster) Krasser, T. princeps (Presl) Gothan, Osmundopsis Harris, Cladophlebis denticulata (Brongniart) Fontaine, C. goeppertianus (Münster) Krasser, C. raciborskii Zeiller, Spiropteris Schimper, Phlebopteris polypodioides Brongniart, Danaeopsis Heer ex Schimper, Thaumatopteris pusilla (Nathorst) Oishi et Yamasita, Dictyophyllum nathorstii Zeiller, D. nilssonii (Brongniart) Goeppert, Clathropteris meniscioides Brongniart, Cl. platyphylla Goeppert, Cl. obovata Oishi, Coniopteris hymenophylloides (Brongniart) Seward, Thinnfeldia Ettingshausen, Augustiphyllum yaobuensis Huang, Scoresbya dentata Harris, Pterophyllum firmifolium Ye, Pt. propinquum Goeppert, Pt. subaequale Hartz, Nilssonia complicatis Li, N. orientalis Heer, N. minor Harris, N. cf. compta (Schenk) Ye, N. cf. polymorpha Schenk, N. pterophylloides Nathorst, N. cf. saighanensis Seward, N. taeniopterioides Halle, N. parabrevis Huang, N. moshanensis Huang, Nilssoniopteris vittata (Brongn.) Florin, Ctenis Lindley et Hutton, Ctenozamites cf. ptilozamioides Zhou, C. cf. cycadea (Berger) Schenk, Cycadolepis corrugata Zeiller, Anomozamites cf. minor Nathorst, A. cf. major (Brong) Huang, A. cf. inconstans (Goeppert) Schimper, A. quadratus Cao, Tyrmia nathorstii (Schenk) Yeh, T. latior Ye, T. lepida Huang, T. susongensis Cao, Otozamites minor Tsao, Ot. hsiangchiensis Sze, Ot. mixomorphus Ye, Ot. tangyanensis Sze, Ptilophyllum hsingshanense (Wu) Cao, Pt. contiguum Sze, Pt. pecten (Philips) Morris, Hsiangchiphyllum trinervis Sze, Ginkgoites cf. tasiakouensis Wu et Li, G. cf. sibiricus (Heer) Seward, G. cf. magnifolius Du Tiot, Baiera cf. furcata (L. et H.) Braun, B. asadai Yabe et Oishi, B. guilhaumatii Zeiller, B. multipartita Sze et Lee, B. cf. gracilis Bunbury, Sphenobaiera huangii (Sze) Hsu ex Li, S. spectabilis (Nath.) Florin, Czekanowskia rigida Heer, C. hartzii Harris, Phoenicopsis Heer, Ginkgodium Yokoyama, Desmiophyllum Lesquereux, Stenorachis (Nathorst) Saporta, Vittifoliolum multinerve Zhou, Pityophyllum longifolium (Nathorst) Möller, Podozamites lanceolatus (L. et H.) Braun, Ferganiella Prynada, Elatocladus Halle, Swedenborgia cryptomerioides Nathorst, Taeniopteris cf. richthofenii (Schenk) Sze, T. inouyei Tateiwa, Conites and Carpolithus (Figure 3—figure supplement 1; Figure 4—figure supplement 1).
Palynological assemblage
Request a detailed protocolPreliminary analysis of the strata yielding Nanjinganthus has recognized abundant palynomorphs. The palynoflora includes Anapiculatisporites sp., Annulispora folliculosa (Rogalska) De Jersey, Contignisporites sp., Cyathidites australis Couper, C. minor Couper, Deltoidospora sp., D. minor Pocock, Dictyophyllidites harrisii Couper, D. mortonii (De Jersey) Playford and Dettmann, Gleicheniidites sp., G. senonicus Ross, Ischyosporites sp., I. variegatus (Couper) Schultz, Leptolepidites verrucatus Couper, Manumia delcourtii (Pocock) Dybkjær, Neoraistrickia ramosus (Balme and Hennelly) Hart, Osmundacidites wellmanii Couper, Polycingulatisporites triangularis (Bolchovitina) Playford and Dettmann, Punctatosporites sp., Retitriletes austroclavatidites (Cookson) Döring et al., R. clavatoides (Couper) Döring et al., Sestrosporites pseudoalveolatus (Couper) Dettmann, Striatella scanica (Nilsson) Filatoff and Price, S. seebergensis Mädler, Alisporites sp., A. robustus Nilsson, Callialasporites dampieri (Balme) Dev, C. minus (Tralau) Guy, C. trilobatus (Balme) Dev, C. turbatus (Balme) Schulz, Cerebropollenites macroverrucosus (Thiergart) Pocock, Chasmatosporites sp., C. apertus (Rogalska) Nilsson, C. hians Nilsson, Classopollis chateaunovi Reyre, C. classoides (Pflug) Pocock and Jansonius, C. meyeriana (Klaus) De Jersey, C. simplex (Danzé-Corsin and Laveine) Reiser and Williams, Cycadopites sp., C. follicularis Wilson and Webster, Ephedripites sp., Monosulcites sp., M. minimus Cookson, Perinopollenites elatoides Couper, Platysaccus sp., Podocarpidites sp., Quadraeculina anellaeformis Maljavkina, Q. enigmata (Couper) Xu and Zhang, Q. minor (Pocock) Xu and Zhang, Spheripollenites psilatus Couper, Vitreisporites pallidus Nilsson (Figure 2—figure supplement 1) (Santos et al., 2018). This palynological assemblage suggests a latest Early Jurassic age for Nanjinganthus.
Isotopic dating
Request a detailed protocolThe samples were processed by crushing, initial heavy liquid and subsequent magnetic separation at Langfang Yuneng Rock Mineral Separation Technology Service Co., Ltd. in Langfang City. More than 1000 grains of zircons were hand-picked under a binocular microscope. More than 200 grains of representative zircons for each sample were coined in epoxy resin mounts, ground and polished to expose the central part of zircons, and then photographed under microscope in transmitting light and reflected light. Afterward, the internal structure of the zircons was studied by means of cathodoluminescence (CL) imaging at the Beijing Gaonianlinghang Technology Co., Ltd. in Beijing City. U-Pb dating of these samples were carried out using laser ablation multicollector inductively coupled plasma mass spectrometry (LA-MC-ICP-MS) at the Tianjin Institute of Geology and Mineral Resources. The laser beam was 35 μm in diameter. Concentrations of U, Th, and Pb elements were calibrated using SRM 610 as the external reference standard. For the analysis method please see refer Li et al., 2009. Repeated analyses of standards yielded precisions at better than 10% for most elements. 207Pb/206Pb, 206Pb/238U, 207Pb/235U and 208Pb/232Th ratios and apparent ages were calculated using ICPMSDataCal software (Liu et al., 2010a; Liu et al., 2010b) and corrected for both instrumental mass bias and depth dependent elemental and isotopic fractionation using zircon GJ-1 as the external standard. U-Pb age Concordia diagram and histograms apparent ages diagram were drawn by using ISOPLOT (ver.3) (Ludwig and Ludwig, 2003).
There was no previous isotopic age for the Xiangshan Group. We sampled the layers above the fossiliferous layers (Figure 1—figure supplement 1) and picked zircon grains for U/Pb dating. The zircon grains appeared to be reworked (Supplementary file 2), with ages ranging from 2738 Ma to 207 Ma (67 zircon grains with the concordance >90% from 168 zircon grains), and 207 Ma (two zircon grains) is the youngest age (Figure 1—figure supplement 1). Most of the zircon grains were of magmatic origin with internal oscillation belts and high Th/U values, implying a granitic provenance. So the upper limit age of Nanjinganthus is 207 Ma (the Late Triassic).
Taking all dating information into consideration, we think that the age of Nanjinganthus falls in the scope ranging from 174 to 207 Ma and is closer to 174 Ma (the latest Early Jurassic). Such a conclusion on absolute age of Nanjinganthus is in agreement with megafossil biostratigraphical analysis (Cao, 1982; Huang, 1983; Huang, 1988; Ju, 1987), although Neocalamites horridus was previously known only in the Late Triassic (Zan et al., 2012).
Materials
The fossils studied here were collected from an outcrop of the South Xiangshan Formation at a quarry owned by the Xiaoyetian Cement Company Ltd. in the northeastern suburb of Nanjing, Jiangsu, China (N32˚08′ 19′′, E118˚58′ 20′′) (Figure 1—figure supplement 1). Plant fossils of the formation have been extensively studied by various scholars (Sze and Chow, 1962; Cao, 1982; Cao, 1998; Cao, 2000; Wang et al., 1982; Huang, 1983; Huang, 1988; Ju, 1987), and our collection from the local outcrop indicates that the fossil plants closely associated with Nanjinganthus constitute a flora dominated by Dipteridaceae (Clathropteris) and various cycadophytes (mainly Nilssonia, Ptilophyllum, and Pterophyllum), which is consistent with previous works. Some of these associated plant fossils are shown in Figure 3—figure supplement 1 and Figure 4—figure supplement 1.
Methods
Request a detailed protocolThe specimens were initially photographed using a Sony ILCE-7 digital camera. The sediment covering the specimens was dégaged using a JUN-AIR pneumatic drill, and the details of the fossils were observed and photographed using a Nikon SMZ1500 stereomicroscope equipped with a Digital Sight DS-Fi1 camera. Organically preserved sepals and petals were processed with 40% peroxide for cuticle analysis according to routine methods, and the processed cuticles and cleaned organic material of the sepals and petals were observed and photographed using the Rhod fluorescent light in a Zeiss Z2 Imager with an AxioCam HRc camera. Extended-focus images were generated using the Z-stack function in an AxioVs40 × 64 V4.9.1.0. The removed cuticles were coated with gold and observed using a Leo 1530 VP scanning electron microscope (SEM), and serial pictures were obtained after the internal details of the flower were exposed through grinding with a pneumatic drill. One of the organically-preserved petals was embedded in resin and sectioned for light microscopy and transmission electron microscopy (TEM). One fragment of the distal portion of a flower embedded in sediments was observed by Micro Computed Laminography (Micro-CL) (Wei et al., 2017) to show the dendroid style embedded in the sediments. All photographs were saved in TIFF format and assembled for publication using Photoshop 7.0.
Data availability
All data generated or analysed during this study are included in the manuscript and supporting files. Source data files have been provided as Supplementary files 1 and 2, Figure 1—figure supplement 1, Figure 2—figure supplement 1, Figure 3–figure supplement 1, Figure 4—figure supplement 1 and Figure 5—figure supplement 1.
References
-
On the Origin of AngiospermsJournal of the Linnean Society of London, Botany 38:29–80.https://doi.org/10.1111/j.1095-8339.1907.tb01074.x
-
The Cactus gynoecium: A new interpretationAmerican Journal of Botany 51:598–610.https://doi.org/10.1002/j.1537-2197.1964.tb06677.x
-
The deepening of Darwin's abominable mysteryNature Ecology & Evolution 1:0169.https://doi.org/10.1038/s41559-017-0169
-
On the occurrence of Scoresbya from Jiangsu and Weichselia from ZhejiangActa Palaeontologica Sinica 21:343–348.
-
A study on the cuticles of some bennettitaleans from the lower part of Xiangshan Group in Jiangsu and Anhui provincesActa Palaeontologica Sinica 37:283–294.
-
Some specimens of gymnospermae from the lower part of Xiangshan Group in Jiangsu and Anhui provinces with study on their cuticulesActa Palaeontologica Sinica 39:334–342.
-
An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IVBotanical Journal of the Linnean Society 181:1–20.https://doi.org/10.1111/boj.12385
-
Establishing a time-scale for plant evolutionNew Phytologist 192:266–301.https://doi.org/10.1111/j.1469-8137.2011.03794.x
-
Phylogenetic Analysis of Seed Plants and the Origin of AngiospermsAnnals of the Missouri Botanical Garden 72:716–793.https://doi.org/10.2307/2399221
-
BookThe Evolution and Classification of Flowering Plants (Second edition)United Kingdom: Thomas Nelson & Sons Ltd.
-
Seed ferns and the origin of angiospermsThe Journal of the Torrey Botanical Society 133:169–209.https://doi.org/10.3159/1095-5674(2006)133[169:SFATOO]2.0.CO;2
-
Preliminary Report of Upper Cretaceous Angiosperm Reproductive Organs From Sweden and Their Level of OrganizationAnnals of the Missouri Botanical Garden 71:403–418.https://doi.org/10.2307/2399032
-
Silvianthemum suecicum gen. et sp. nov., a new saxifragalean flower from the late Cretaceous of SwedenBiologiske Skrifter 36:1–17.
-
BookThe Early Flowers and Angiosperm EvolutionCambridge: Cambridge University Press.https://doi.org/10.1017/CBO9780511980206
-
A new angiosperm genus from the Lower Cretaceous Yixian Formation, Western Liaoning, ChinaActa Geologica Sinica 87:916–925.https://doi.org/10.1111/1755-6724.12100
-
A whole plant herbaceous angiosperm from the Middle Jurassic of ChinaActa Geologica Sinica 90:19–29.https://doi.org/10.1111/1755-6724.12592
-
A Dichocarpum-like angiosperm from the Early Cretaceous of ChinaActa Geologica Sinica 90:1–8.https://doi.org/10.1111/1755-6724.13059
-
A new member of the CaytonialesThe New Phytologist 32:97–114.https://doi.org/10.1111/j.1469-8137.1933.tb07001.x
-
A boreal early cradle of Angiosperms? Angiosperm-like pollen from the Middle Triassic of the Barents Sea (Norway)Journal of Micropalaeontology 23:97–104.https://doi.org/10.1144/jm.23.2.97
-
Geology of Chung Shan and its bearing on the supply of artesian water in NanjingBulletin of the Geological Society of China 7:139–156.https://doi.org/10.1111/j.1755-6724.1928.mp7002002.x
-
The Early Jurassic Xiangshan Flora from the Yangzi River Valley in Anhui province of eastern ChinaEarth Science - Journal of Wuhan College of Geology 20:25–36.
-
Vertical diversities of the Early Jurassic plant fossils in the middle-lower Changjiang valleyGeological Review 34:193–202.
-
Subdivision of the Lower-Middle Jurassic strata in south JiangsuBulletin of Nanjing Institute of Geology and Mineral Resources, Chinese Academy of Geological Sciences 8:33–44.
-
BookPlant Systematics: a Phylogenetic ApproachSunderland, MA: Sinauer Associate Inc.
-
Angiosperm leaves associated with Sinocarpus infructescences from the Yixian Formation (mid-Early Cretaceous) of NE ChinaPlant Systematics and Evolution 262:173–187.https://doi.org/10.1007/s00606-006-0461-6
-
The study of determination of zircon ages using LA-MC-ICP-MSActa Mineralogica Sinica 29:600–601.
-
Preliminary report on the geology and the mineral resources of KiangsuMemoir of the Geological Survey of China 4:1–34.
-
Reappraisement and refinement of zircon U-Pb isotope and trace element analyses by LA-ICP-MSChinese Science Bulletin 55:1535–1546.
-
A perfect flower from the Jurassic of ChinaHistorical Biology 28:707–719.https://doi.org/10.1080/08912963.2015.1020423
-
Zhangwuia: an enigmatic organ with a bennettitalean appearance and enclosed ovulesEarth and Environmental Science Transactions of the Royal Society of Edinburgh 54:1–10.https://doi.org/10.1017/S1755691018000257
-
A novel angiosperm from the Early Cretaceous and its implications for carpel-derivingActa Geologica Sinica 92:1293–1298.https://doi.org/10.1111/1755-6724.13627
-
A Geochronological Toolkit for Microsoft ExcelUser's manual for isoplot 3.00: , A Geochronological Toolkit for Microsoft Excel, Berkeley, Berkeley Geochronology Center Special Publication.
-
A Reevaluation of Seed Plant PhylogenyAnnals of the Missouri Botanical Garden 81:484–533.https://doi.org/10.2307/2399901
-
BookDas suedoestliche China. Allgemeine UebersichtIn: Tiessen E, editors. China. Ergebnisse Eigener Reisen Und Darauf Gegruendeter Studien, 3. Berlin: Verlag von Dietrich Reimer (Ernst Vohsen). pp. 395–753.
-
Is the anthophyte hypothesis alive and well? New evidence from the reproductive structures of BennettitalesAmerican Journal of Botany 96:296–322.https://doi.org/10.3732/ajb.0800209
-
Development and evolution of basal cauline placentation: basella rubraAmerican Journal of Botany 75:918–927.https://doi.org/10.1002/j.1537-2197.1988.tb13516.x
-
The ancestral flower of angiosperms and its early diversificationNature Communications 8:16047.https://doi.org/10.1038/ncomms16047
-
Male cone evolution in Conifers: Not all that simpleAmerican Journal of Plant Sciences 5:2842–2857.https://doi.org/10.4236/ajps.2014.518300
-
Die Räto-Jurassischen floren des Iran und Afghanistans. 4. Die Rätische zwitterblüte Irania hermphroditic nov. spec. und ihre bedeutung für die Phylogenie der AngiospermenPaläontographica B 161:98–145.
-
BookEarly Angiosperms and Their Associated Plants From Western Liaoning, ChinaShanghai: Shanghai Technology & Education Press.
-
Angiosperm ovule and carpels: their characters and polarities, distribution in basal clades, and structural evolutionPostilla 208:1–40.
-
BookThe origin and evolution of the angiosperm carpelIn: Kirchner G, Taylor DWHickey LJ, editors. Flowering Plant Origin, Evolution & Phylogeny. New York: Chapman. pp. 116–140.
-
BookPaleobotany: The Biology and Evolution of Fossil Plants (2nd ed)Amsterdam: Elsevier.
-
The Caytoniales, a New Group of Angiospermous Plants from the Jurassic Rocks of YorkshirePhilosophical Transactions of the Royal Society B: Biological Sciences 213:299–363.https://doi.org/10.1098/rstb.1925.0006
-
Seed cone structure in conifers in relation to development and pollination: a biological approachCanadian Journal of Botany 80:1250–1273.https://doi.org/10.1139/b02-112
-
Schmeissneria: a missing link to angiosperms?BMC Evolutionary Biology 7:14.https://doi.org/10.1186/1471-2148-7-14
-
The earliest normal flower from Liaoning Province, ChinaJournal of Integrative Plant Biology 51:800–811.https://doi.org/10.1111/j.1744-7909.2009.00838.x
-
Schmeissneria: An angiosperm from the Early JurassicJournal of Systematics and Evolution 48:326–335.https://doi.org/10.1111/j.1759-6831.2010.00090.x
-
The earliest ascidiate carpel and its implications for angiosperm evolutionActa Geologica Sinica - English Edition 85:998–1002.https://doi.org/10.1111/j.1755-6724.2011.00534.x
-
Xingxueanthus: An enigmatic Jurassic seed plant and its implications for the origin of angiospermyActa Geologica Sinica - English Edition 84:47–55.https://doi.org/10.1111/j.1755-6724.2010.00169.x
-
The megafossil record of early angiosperms in China since 1930sHistorical Biology 27:398–404.https://doi.org/10.1080/08912963.2014.889695
-
A micro-CL system and its applicationsReview of Scientific Instruments 88:115107.https://doi.org/10.1063/1.4989444
-
Dinosaur-associated Poaceae epidermis and phytoliths from the Early Cretaceous of ChinaNational Science Review 5:721–727.https://doi.org/10.1093/nsr/nwx145
-
ConferenceA palaeobotanical approach to the classification, correlation and geological ages of the non-marine Mesozoic deposits of ChinaScientific Papers on Geology for International Exchange Prepared for the 26th International Geological Congress 4. Stratigraphy and Palaeontology. pp. 82–91.
Article and author information
Author details
Funding
National Natural Science Foundation of China (41688103)
- Xin Wang
Chinese Academy of Sciences (XDPB26000000)
- Xin Wang
National Natural Science Foundation of China (91514302)
- Xin Wang
National Natural Science Foundation of China (41572046)
- Xin Wang
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Acknowledgements
We thank Ms Huijun Ma, Brandon Y. Wang, and Mingzhi Fu for their help collecting the valuable specimens, Ms. Chunzhao Wang for help with SEM during this research, and Dr. Walter Judd at the University of Florida for comments and suggestions. This research was supported by the Strategic Priority Research Program of Chinese Academy of Sciences (Grant No. XDB26000000), the National Natural Science Foundation of China (41688103, 91514302, 41572046) awarded to XW. This is a contribution to UNESCO IGCP632. We declare no competing interests. We appreciate the kind and constructive helps from Dr. David Taylor, Hongqi Li, Ian Baldwin and an anonymous reviewer, who made our manuscript much improved.
Copyright
© 2018, Fu et al.
This article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use and redistribution provided that the original author and source are credited.
Metrics
-
- 13,517
- views
-
- 1,552
- downloads
-
- 41
- citations
Views, downloads and citations are aggregated across all versions of this paper published by eLife.
Citations by DOI
-
- 41
- citations for umbrella DOI https://doi.org/10.7554/eLife.38827
Download links
Downloads (link to download the article as PDF)
Open citations (links to open the citations from this article in various online reference manager services)
Cite this article (links to download the citations from this article in formats compatible with various reference manager tools)
Further reading
-
- Evolutionary Biology
eLife has published papers on topics as diverse as paleoproteomics, ancient insects and the discovery of a new hominin species.
-
Fossils found in China suggest that the first flowers emerged much earlier than previously thought.






